Impact of Antibodies and Strain Polymorphisms on Cytomegalovirus Entry and Spread in Fibroblasts and Epithelial Cells
- PMID: 28381568
- PMCID: PMC5469265
- DOI: 10.1128/JVI.01650-16
Impact of Antibodies and Strain Polymorphisms on Cytomegalovirus Entry and Spread in Fibroblasts and Epithelial Cells
Abstract
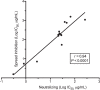
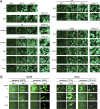
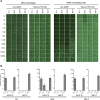
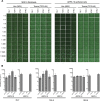
Cytomegalovirus (CMV) entry into fibroblasts differs from entry into epithelial cells. CMV also spreads cell to cell and can induce syncytia. To gain insights into these processes, 27 antibodies targeting epitopes in CMV virion glycoprotein complexes, including glycoprotein B (gB), gH/gL, and the pentamer, were evaluated for their effects on viral entry and spread. No antibodies inhibited CMV spread in fibroblasts, including those with potent neutralizing activity against fibroblast entry, while all antibodies that neutralized epithelial cell entry also inhibited spread in epithelial cells and a correlation existed between the potencies of these two activities. This suggests that exposure of virions to the cell culture medium is obligatory during spread in epithelial cells but not in fibroblasts. In fibroblasts, the formation of syncytiumlike structures was impaired not only by antibodies to gB or gH/gL but also by antibodies to the pentamer, suggesting a potential role for the pentamer in promoting fibroblast fusion. Four antibodies reacted with linear epitopes near the N terminus of gH, exhibited strain specificity, and neutralized both epithelial cell and fibroblast entry. Five other antibodies recognized conformational epitopes in gH/gL and neutralized both fibroblast and epithelial cell entry. That these antibodies were strain specific for neutralizing fibroblast but not epithelial cell entry suggests that polymorphisms external to certain gH/gL epitopes may influence antibody neutralization during fibroblast but not epithelial cell entry. These findings may have implications for elucidating the mechanisms of CMV entry, spread, and antibody evasion and may assist in determining which antibodies may be most efficacious following active immunization or passive administration.IMPORTANCE Cytomegalovirus (CMV) is a significant cause of birth defects among newborns infected in utero and morbidity and mortality in transplant and AIDS patients. Monoclonal antibodies and vaccines targeting humoral responses are under development for prophylactic or therapeutic use. The findings reported here (i) confirm that cell-to-cell spread of CMV is sensitive to antibody inhibition in epithelial cells but not fibroblasts, (ii) demonstrate that antibodies can restrict the formation in vitro of syncytiumlike structures that resemble syncytial cytomegalic cells that are associated with CMV disease in vivo, and (iii) reveal that neutralization of CMV by antibodies to certain epitopes in gH or gH/gL is both strain and cell type dependent and can be governed by polymorphisms in sequences external to the epitopes. These findings serve to elucidate the mechanisms of CMV entry, spread, and antibody evasion and may have important implications for the development of CMV vaccines and immunotherapeutics.
Keywords: antibodies; cytomegalovirus; fusion; neutralization; neutralizing antibodies; polymorphisms; spread; virus entry.
Copyright © 2017 American Society for Microbiology.
Figures



Similar articles
-
Monoclonal Antibodies to Different Components of the Human Cytomegalovirus (HCMV) Pentamer gH/gL/pUL128L and Trimer gH/gL/gO as well as Antibodies Elicited during Primary HCMV Infection Prevent Epithelial Cell Syncytium Formation.J Virol. 2016 Jun 24;90(14):6216-6223. doi: 10.1128/JVI.00121-16. Print 2016 Jul 15. J Virol. 2016. PMID: 27122579 Free PMC article.
-
Comparative neutralizing potencies of antibodies suggest conservation as well as mechanistic differences in human cytomegalovirus entry into epithelial and endothelial cells.Virol J. 2020 Apr 8;17(1):50. doi: 10.1186/s12985-020-01320-2. Virol J. 2020. PMID: 32268919 Free PMC article.
-
Cytomegalovirus Virions Shed in Urine Have a Reversible Block to Epithelial Cell Entry and Are Highly Resistant to Antibody Neutralization.Clin Vaccine Immunol. 2017 Jun 5;24(6):e00024-17. doi: 10.1128/CVI.00024-17. Print 2017 Jun. Clin Vaccine Immunol. 2017. PMID: 28404573 Free PMC article.
-
Pathogen at the Gates: Human Cytomegalovirus Entry and Cell Tropism.Viruses. 2018 Dec 11;10(12):704. doi: 10.3390/v10120704. Viruses. 2018. PMID: 30544948 Free PMC article. Review.
-
Virion Glycoprotein-Mediated Immune Evasion by Human Cytomegalovirus: a Sticky Virus Makes a Slick Getaway.Microbiol Mol Biol Rev. 2016 Jun 15;80(3):663-77. doi: 10.1128/MMBR.00018-16. Print 2016 Sep. Microbiol Mol Biol Rev. 2016. PMID: 27307580 Free PMC article. Review.
Cited by
-
A Replication-Defective Human Cytomegalovirus Vaccine Elicits Humoral Immune Responses Analogous to Those with Natural Infection.J Virol. 2019 Nov 13;93(23):e00747-19. doi: 10.1128/JVI.00747-19. Print 2019 Dec 1. J Virol. 2019. PMID: 31511385 Free PMC article. Clinical Trial.
-
The N Terminus of Human Cytomegalovirus Glycoprotein O Is Important for Binding to the Cellular Receptor PDGFRα.J Virol. 2019 May 15;93(11):e00138-19. doi: 10.1128/JVI.00138-19. Print 2019 Jun 1. J Virol. 2019. PMID: 30894468 Free PMC article.
-
Inhibition of human cytomegalovirus entry into mucosal epithelial cells.Antiviral Res. 2024 Oct;230:105971. doi: 10.1016/j.antiviral.2024.105971. Epub 2024 Jul 27. Antiviral Res. 2024. PMID: 39074588
-
Inclusion of Antibodies to Cell Culture Media Preserves the Integrity of Genes Encoding RL13 and the Pentameric Complex Components During Fibroblast Passage of Human Cytomegalovirus.Viruses. 2019 Mar 5;11(3):221. doi: 10.3390/v11030221. Viruses. 2019. PMID: 30841507 Free PMC article.
-
Cell Fusion and Syncytium Formation in Betaherpesvirus Infection.Viruses. 2021 Sep 30;13(10):1973. doi: 10.3390/v13101973. Viruses. 2021. PMID: 34696402 Free PMC article. Review.
References
-
- Adler SP, Starr SE, Plotkin SA, Hempfling SH, Buis J, Manning ML, Best AM. 1995. Immunity induced by primary human cytomegalovirus infection protects against secondary infection among women of childbearing age. J Infect Dis 171:26–32. doi:10.1093/infdis/171.1.26 (Erratum, 171: 1080, doi:10.1093/infdis/171.4.1080-a.) - DOI - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

