Percutaneous Autologous Bone Marrow-Derived Mesenchymal Stromal Cell Implantation Is Safe for Reconstruction of Human Lower Limb Long Bone Atrophic Nonunion
- PMID: 28367426
- PMCID: PMC5241512
- DOI: 10.22074/cellj.2016.4866
Percutaneous Autologous Bone Marrow-Derived Mesenchymal Stromal Cell Implantation Is Safe for Reconstruction of Human Lower Limb Long Bone Atrophic Nonunion
Abstract
Objective: Nonunion is defined as a minimum of a 9-month period of time since an injury with no visibly progressive signs of healing for 3 months. Recent studies show that application of mesenchymal stromal cells (MSCs) in the laboratory setting is effective for bone regeneration. Animal studies have shown that MSCs can be used to treat nonunions. For the first time in an Iranian population, the present study investigated the safety of MSC implantation to treat human lower limb long bone nonunion.
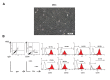
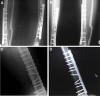
Materials and methods: It is a prospective clinical trial for evaluating the safety of using autologus bone marrow derived mesenchymal stromal cells for treating nonunion. Orthopedic surgeons evaluated 12 patients with lower limb long bone nonunion for participation in this study. From these, 5 complied with the eligibility criteria and received MSCs. Under fluoroscopic guidance, patients received a one-time implantation of 20-50×106 MSCs into the nonunion site. All patients were followed by anterior-posterior and lateral X-rays from the affected limb, in addition to hematological, biochemical, and serological laboratory tests obtained before and 1, 3, 6, and 12 months after the implantation. Possible adverse effects that included local or systemic, serious or non-serious, and related or unrelated effects were recorded during this time period.
Results: From a safety perspective, all patients tolerated the MSCs implantation during the 12 months of the trial. Three patients had evidence of bony union based on the after implantation Xrays.
Conclusion: The results have suggested that implantation of bone marrow-derived MSCs is a safe treatment for nonunion. A double-blind, controlled clinical trial is required to assess the efficacy of this treatment (Registration Number: NCT01206179).
Keywords: Autologous; Bone Marrow; Mesenchymal Stromal Cells; Nonunion.
Figures
Similar articles
-
Mesenchymal Stromal Cells Implantation in Combination with Platelet Lysate Product Is Safe for Reconstruction of Human Long Bone Nonunion.Cell J. 2016 Fall;18(3):302-309. doi: 10.22074/cellj.2016.4557. Epub 2016 Aug 24. Cell J. 2016. PMID: 27602311 Free PMC article.
-
Efficacy and safety of autologous or allogeneic mesenchymal stromal cells from adult adipose tissue expanded and combined with tricalcium phosphate biomaterial for the surgical treatment of atrophic nonunion of long bones: a phase II clinical trial.J Transl Med. 2024 May 24;22(1):493. doi: 10.1186/s12967-024-05280-x. J Transl Med. 2024. PMID: 38789992 Free PMC article. Clinical Trial.
-
Intra-articular knee implantation of autologous bone marrow-derived mesenchymal stromal cells in rheumatoid arthritis patients with knee involvement: Results of a randomized, triple-blind, placebo-controlled phase 1/2 clinical trial.Cytotherapy. 2018 Apr;20(4):499-506. doi: 10.1016/j.jcyt.2017.12.009. Epub 2018 Feb 7. Cytotherapy. 2018. PMID: 29428486 Clinical Trial.
-
Mesenchymal stem cell implantation in atrophic nonunion of the long bones: A translational study.Bone Joint Res. 2016 Jul;5(7):287-93. doi: 10.1302/2046-3758.57.2000587. Bone Joint Res. 2016. PMID: 27412657 Free PMC article.
-
Efficacy and Safety of Autologous Cell-based Therapy in Patients with No-option Critical Limb Ischaemia: A Meta-Analysis.Curr Stem Cell Res Ther. 2018;13(4):265-283. doi: 10.2174/1574888X13666180313141416. Curr Stem Cell Res Ther. 2018. PMID: 29532760 Review.
Cited by
-
The combination of a 3D-Printed porous Ti-6Al-4V alloy scaffold and stem cell sheet technology for the construction of biomimetic engineered bone at an ectopic site.Mater Today Bio. 2022 Sep 15;16:100433. doi: 10.1016/j.mtbio.2022.100433. eCollection 2022 Dec. Mater Today Bio. 2022. PMID: 36157052 Free PMC article.
-
Cell-based therapy in the treatment of musculoskeletal diseases.Stem Cells Transl Med. 2024 Oct 10;13(10):959-978. doi: 10.1093/stcltm/szae049. Stem Cells Transl Med. 2024. PMID: 39226104 Free PMC article. Review.
-
Organ-Specific Differentiation of Human Adipose-Derived Stem Cells in Various Organs of Xenotransplanted Rats: A Pilot Study.Life (Basel). 2022 Jul 25;12(8):1116. doi: 10.3390/life12081116. Life (Basel). 2022. PMID: 35892918 Free PMC article.
-
Systematic review assessing the evidence for the use of stem cells in fracture healing.Bone Jt Open. 2020 Oct 6;1(10):628-638. doi: 10.1302/2633-1462.110.BJO-2020-0129. eCollection 2020 Oct. Bone Jt Open. 2020. PMID: 33215094 Free PMC article.
-
Exosomes in craniofacial tissue reconstruction.Maxillofac Plast Reconstr Surg. 2022 Aug 24;44(1):27. doi: 10.1186/s40902-022-00357-3. Maxillofac Plast Reconstr Surg. 2022. PMID: 35999408 Free PMC article. Review.
References
-
- Einhorn TA. Enhancement of fracture-healing. J Bone Joint Surg Am. 1995;77(6):940–956. - PubMed
-
- Giannoudis PV, Einhorn TA, Marsh D. Fracture healing: the diamond concept. Injury. 2007;38(Suppl 4):S3–6. - PubMed
-
- Tressler MA, Richards JE, Sofianos D, Comrie FK, Kregor PJ, Obremskey WT. Bone morphogenetic protein-2 compared to autologous iliac crest bone graft in the treatment of long bone nonunion. Orthopedics. 2011;34(12):e877–884. - PubMed
-
- Khan Y, Laurencin CT. Fracture repair with ultrasound: clinical and cell-based evaluation. J Bone Joint Surg Am. 2008;90(Suppl 1):138–144. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
