Exosomes Isolated from Ascites of T-Cell Lymphoma-Bearing Mice Expressing Surface CD24 and HSP-90 Induce a Tumor-Specific Immune Response
- PMID: 28360912
- PMCID: PMC5352668
- DOI: 10.3389/fimmu.2017.00286
Exosomes Isolated from Ascites of T-Cell Lymphoma-Bearing Mice Expressing Surface CD24 and HSP-90 Induce a Tumor-Specific Immune Response
Abstract
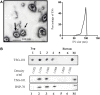
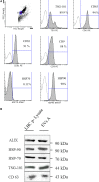
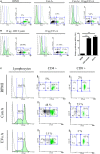
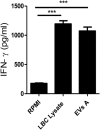
Extracellular vesicles (EVs), including endosome-derived nanovesicles (exosomes), are involved in cell-cell communication. Through transfer of their molecular contents, extracellular nanovesicles can alter the function of recipient cells. Due to these characteristics, EVs have shown potential as a new alternative for cancer immunotherapy. Tumor exosomes isolated from malignant ascites can activate dendritic cells, thereby priming the immune system to recognize and kill cancer cells. However, a suppressive role on tumor immune response has also been reported, suggesting that the neoplastic stage of carcinogenesis and the microenvironment where tumor cells grow may influence the amount of EVs released by the cell. This neoplastic stage and microenvironment may also impact EVs' components such as proteins and miRNA, determining their biological behavior. Most T-cell lymphomas have an aggressive clinical course and poor prognosis. Consequently, complementary alternative therapies are needed to improve the survival rates achieved with conventional treatments. In this work, we have characterized EVs isolated from ascites of mice bearing a very aggressive murine T-cell lymphoma and have studied their immunogenic properties. Small EVs were isolated by differential centrifugation, ultrafiltration, and ultracentrifugation at 100,000 × g on a sucrose cushion. The EVs were defined as exosomes by their morphology and size analyzed by electron microscopy, their floating density on a sucrose gradient, as well as their expression of endosome marker proteins ALIX, TSG-101; the tetraspanins CD63, CD9, and CD81. In addition, they contain tumor antigens, the marker for malignancy CD24, the heat shock protein HSP-70, and an unusual surface expression of HSP-90 was demonstrated. The administration of EVs isolated from ascites (EVs A) into naïve-syngeneic mice induced both humoral and cellular immune responses that allowed the rejection of subsequent tumor challenges. However, the immunization had no effect on a non-related mammary adenocarcinoma, demonstrating that the immune response elicited was specific and also it induced immune memory. In vitro analysis demonstrated that T-cells from EVs A-immunized mice secrete IFN-γ in response to tumor stimulation. Furthermore, tumor-specific CD4+ and CD8+ IFN-γ secreting cells could be efficiently expanded from mice immunized with EVs A, showing that a T helper 1 response is involved in tumor rejection. Our findings confirm exosomes as promising defined acellular tumor antigens for the development of an antitumor vaccine.
Keywords: T-cell lymphoma; ascites; exosomes; immune response; tumor vaccine.
Figures


Similar articles
-
Cyclophosphamide enhances the release of tumor exosomes that elicit a specific immune response in vivo in a murine T-cell lymphoma.Vaccine. 2019 Mar 14;37(12):1565-1576. doi: 10.1016/j.vaccine.2019.02.004. Epub 2019 Feb 15. Vaccine. 2019. PMID: 30777349
-
Highly-purified exosomes and shed microvesicles isolated from the human colon cancer cell line LIM1863 by sequential centrifugal ultrafiltration are biochemically and functionally distinct.Methods. 2015 Oct 1;87:11-25. doi: 10.1016/j.ymeth.2015.04.008. Epub 2015 Apr 16. Methods. 2015. PMID: 25890246
-
Tumor necrosis factor gene-engineered J558 tumor cell-released exosomes stimulate tumor antigen P1A-specific CD8+ CTL responses and antitumor immunity.Cancer Biother Radiopharm. 2010 Feb;25(1):21-8. doi: 10.1089/cbr.2009.0714. Cancer Biother Radiopharm. 2010. PMID: 20187793
-
Exosomes and their roles in immune regulation and cancer.Semin Cell Dev Biol. 2015 Apr;40:72-81. doi: 10.1016/j.semcdb.2015.02.009. Epub 2015 Feb 25. Semin Cell Dev Biol. 2015. PMID: 25724562 Review.
-
Immune Regulation by Dendritic Cell Extracellular Vesicles in Cancer Immunotherapy and Vaccines.Cancers (Basel). 2020 Nov 28;12(12):3558. doi: 10.3390/cancers12123558. Cancers (Basel). 2020. PMID: 33260499 Free PMC article. Review.
Cited by
-
Nanotechnology for the Development of Nanovaccines in Cancer Immunotherapy.Adv Exp Med Biol. 2021;1295:303-315. doi: 10.1007/978-3-030-58174-9_13. Adv Exp Med Biol. 2021. PMID: 33543465
-
Tumor-Derived Microvesicles Enhance Cross-Processing Ability of Clinical Grade Dendritic Cells.Front Immunol. 2018 Nov 5;9:2481. doi: 10.3389/fimmu.2018.02481. eCollection 2018. Front Immunol. 2018. PMID: 30455687 Free PMC article.
-
Knock-Down of CD24 in Astrocytes Aggravates Oxyhemoglobin-Induced Hippocampal Neuron Impairment.Neurochem Res. 2022 Mar;47(3):590-600. doi: 10.1007/s11064-021-03468-x. Epub 2021 Oct 19. Neurochem Res. 2022. PMID: 34665391
-
The Origin and Functions of Exosomes in Cancer.Front Oncol. 2018 Mar 20;8:66. doi: 10.3389/fonc.2018.00066. eCollection 2018. Front Oncol. 2018. PMID: 29616188 Free PMC article. Review.
-
Exosomes in Immune Regulation.Noncoding RNA. 2021 Jan 8;7(1):4. doi: 10.3390/ncrna7010004. Noncoding RNA. 2021. PMID: 33435564 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

