The cell cycle checkpoint inhibitors in the treatment of leukemias
- PMID: 28356161
- PMCID: PMC5371185
- DOI: 10.1186/s13045-017-0443-x
The cell cycle checkpoint inhibitors in the treatment of leukemias
Abstract
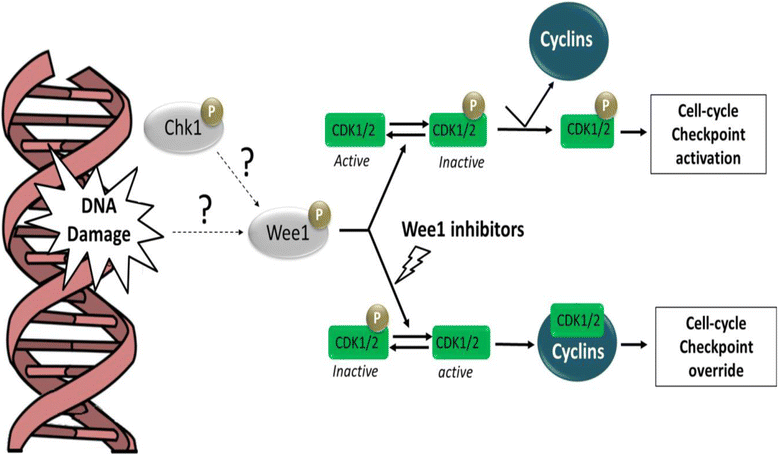
The inhibition of the DNA damage response (DDR) pathway in the treatment of cancers has recently reached an exciting stage with several cell cycle checkpoint inhibitors that are now being tested in several clinical trials in cancer patients. Although the great amount of pre-clinical and clinical data are from the solid tumor experience, only few studies have been done on leukemias using specific cell cycle checkpoint inhibitors. This review aims to summarize the most recent data found on the biological mechanisms of the response to DNA damages highlighting the role of the different elements of the DDR pathway in normal and cancer cells and focusing on the main genetic alteration or aberrant gene expression that has been found on acute and chronic leukemias. This review, for the first time, outlines the most important pre-clinical and clinical data available on the efficacy of cell cycle checkpoint inhibitors in single agent and in combination with different agents normally used for the treatment of acute and chronic leukemias.
Keywords: Acute lymphoblastic leukemia; Acute myeloid leukemia; Checkpoint kinase inhibitor; Chronic lymphocytic leukemia; Chronic myeloid leukemia; DNA damage response.
Figures
Similar articles
-
ATR-CHK1 pathway as a therapeutic target for acute and chronic leukemias.Cancer Treat Rev. 2020 Aug;88:102026. doi: 10.1016/j.ctrv.2020.102026. Epub 2020 May 16. Cancer Treat Rev. 2020. PMID: 32592909 Review.
-
Re-purposing clinical kinase inhibitors to enhance chemosensitivity by overriding checkpoints.Cell Cycle. 2014;13(14):2172-91. doi: 10.4161/cc.29214. Epub 2014 Jun 23. Cell Cycle. 2014. PMID: 24955955 Free PMC article.
-
A regimen combining the Wee1 inhibitor AZD1775 with HDAC inhibitors targets human acute myeloid leukemia cells harboring various genetic mutations.Leukemia. 2015 Apr;29(4):807-18. doi: 10.1038/leu.2014.296. Epub 2014 Oct 6. Leukemia. 2015. PMID: 25283841 Free PMC article.
-
Inhibition of WEE1 kinase and cell cycle checkpoint activation sensitizes head and neck cancers to natural killer cell therapies.J Immunother Cancer. 2018 Jun 21;6(1):59. doi: 10.1186/s40425-018-0374-2. J Immunother Cancer. 2018. PMID: 29925431 Free PMC article.
-
Targeting PARP proteins in acute leukemia: DNA damage response inhibition and therapeutic strategies.J Hematol Oncol. 2022 Jan 22;15(1):10. doi: 10.1186/s13045-022-01228-0. J Hematol Oncol. 2022. PMID: 35065680 Free PMC article. Review.
Cited by
-
Targeted Osmotic Lysis: A Novel Approach to Targeted Cancer Therapies.Biomedicines. 2022 Apr 2;10(4):838. doi: 10.3390/biomedicines10040838. Biomedicines. 2022. PMID: 35453588 Free PMC article. Review.
-
Strategies For Targeting Chronic Myeloid Leukaemia Stem Cells.Blood Lymphat Cancer. 2019 Nov 6;9:45-52. doi: 10.2147/BLCTT.S228815. eCollection 2019. Blood Lymphat Cancer. 2019. PMID: 31807112 Free PMC article. Review.
-
Upregulation of Cyclin B2 (CCNB2) in breast cancer contributes to the development of lymphovascular invasion.Am J Cancer Res. 2022 Feb 15;12(2):469-489. eCollection 2022. Am J Cancer Res. 2022. PMID: 35261781 Free PMC article.
-
SETD8, a frequently mutated gene in cervical cancer, enhances cisplatin sensitivity by impairing DNA repair.Cell Biosci. 2023 Jun 12;13(1):107. doi: 10.1186/s13578-023-01054-y. Cell Biosci. 2023. PMID: 37308924 Free PMC article.
-
Sirtuin inhibition and anti-cancer activities of ethyl 2-benzimidazole-5-carboxylate derivatives.Medchemcomm. 2019 Nov 18;10(12):2140-2145. doi: 10.1039/c9md00323a. eCollection 2019 Dec 1. Medchemcomm. 2019. PMID: 32904142 Free PMC article.
References
-
- Sallmyr A, Fan J, Rassool FV. Genomic instability in myeloid malignancies: increased reactive oxygen species (ROS), DNA double strand breaks (DSBs) and error-prone repair. Cancer Lett. 2008;1–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

