Lack of IL-1R8 in neurons causes hyperactivation of IL-1 receptor pathway and induces MECP2-dependent synaptic defects
- PMID: 28347403
- PMCID: PMC5370184
- DOI: 10.7554/eLife.21735
Lack of IL-1R8 in neurons causes hyperactivation of IL-1 receptor pathway and induces MECP2-dependent synaptic defects
Abstract
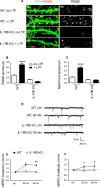
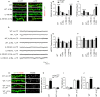
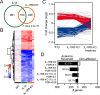
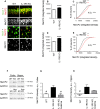
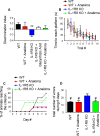
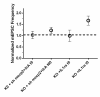
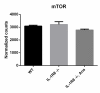
Inflammation modifies risk and/or severity of a variety of brain diseases through still elusive molecular mechanisms. Here we show that hyperactivation of the interleukin 1 pathway, through either ablation of the interleukin 1 receptor 8 (IL-1R8, also known as SIGIRR or Tir8) or activation of IL-1R, leads to up-regulation of the mTOR pathway and increased levels of the epigenetic regulator MeCP2, bringing to disruption of dendritic spine morphology, synaptic plasticity and plasticity-related gene expression. Genetic correction of MeCP2 levels in IL-1R8 KO neurons rescues the synaptic defects. Pharmacological inhibition of IL-1R activation by Anakinra corrects transcriptional changes, restores MeCP2 levels and spine plasticity and ameliorates cognitive defects in IL-1R8 KO mice. By linking for the first time neuronal MeCP2, a key player in brain development, to immune activation and demonstrating that synaptic defects can be pharmacologically reversed, these data open the possibility for novel treatments of neurological diseases through the immune system modulation.
Keywords: MeCP2; Neuroimmunology; neuroscience; synaptic plasticity.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures

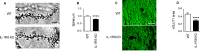
Similar articles
-
IL-37 requires the receptors IL-18Rα and IL-1R8 (SIGIRR) to carry out its multifaceted anti-inflammatory program upon innate signal transduction.Nat Immunol. 2015 Apr;16(4):354-65. doi: 10.1038/ni.3103. Epub 2015 Mar 2. Nat Immunol. 2015. PMID: 25729923
-
Expression and function of IL-1R8 (TIR8/SIGIRR): a regulatory member of the IL-1 receptor family in platelets.Cardiovasc Res. 2016 Sep;111(4):373-84. doi: 10.1093/cvr/cvw162. Epub 2016 Jun 13. Cardiovasc Res. 2016. PMID: 27297888
-
Characterization of a long isoform of IL-1R8 (TIR8/SIGIRR).Eur Cytokine Netw. 2017 Jun 1;28(2):63-69. doi: 10.1684/ecn.2017.0395. Eur Cytokine Netw. 2017. PMID: 28840837
-
TIR8/SIGIRR: an IL-1R/TLR family member with regulatory functions in inflammation and T cell polarization.Trends Immunol. 2009 Sep;30(9):439-46. doi: 10.1016/j.it.2009.06.001. Epub 2009 Aug 21. Trends Immunol. 2009. PMID: 19699681 Review.
-
IL-1R8: A molecular brake of anti-tumor and anti-viral activity of NK cells and ILC.Semin Immunol. 2023 Mar;66:101712. doi: 10.1016/j.smim.2023.101712. Epub 2023 Feb 6. Semin Immunol. 2023. PMID: 36753974 Review.
Cited by
-
Neurological consequences of neurovascular unit and brain vasculature damages: potential risks for pregnancy infections and COVID-19-babies.FEBS J. 2022 Jun;289(12):3374-3392. doi: 10.1111/febs.16020. Epub 2021 May 26. FEBS J. 2022. PMID: 33998773 Free PMC article. Review.
-
Molecular Mechanisms in the Genesis of Seizures and Epilepsy Associated With Viral Infection.Front Mol Neurosci. 2022 May 9;15:870868. doi: 10.3389/fnmol.2022.870868. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35615063 Free PMC article. Review.
-
Col4a2 Mutations Contribute to Infantile Epileptic Spasm Syndrome and Neuroinflammation.Int J Med Sci. 2024 Jul 2;21(9):1756-1768. doi: 10.7150/ijms.97164. eCollection 2024. Int J Med Sci. 2024. PMID: 39006838 Free PMC article.
-
Principles of inflammasome priming and inhibition: Implications for psychiatric disorders.Brain Behav Immun. 2018 Oct;73:66-84. doi: 10.1016/j.bbi.2018.06.010. Epub 2018 Jun 11. Brain Behav Immun. 2018. PMID: 29902514 Free PMC article. Review.
-
The intellectual base and research fronts of IL-37: A bibliometric review of the literature from WoSCC.Front Immunol. 2022 Jul 22;13:931783. doi: 10.3389/fimmu.2022.931783. eCollection 2022. Front Immunol. 2022. PMID: 35935954 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

