Inhibition of serum and glucocorticoid regulated kinase-1 as novel therapy for cardiac arrhythmia disorders
- PMID: 28336914
- PMCID: PMC5428512
- DOI: 10.1038/s41598-017-00413-3
Inhibition of serum and glucocorticoid regulated kinase-1 as novel therapy for cardiac arrhythmia disorders
Abstract
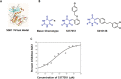
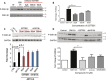
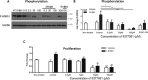
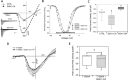
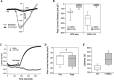
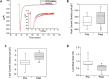
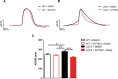
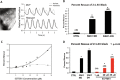
Alterations in sodium flux (INa) play an important role in the pathogenesis of cardiac arrhythmias and may also contribute to the development of cardiomyopathies. We have recently demonstrated a critical role for the regulation of the voltage-gated sodium channel NaV1.5 in the heart by the serum and glucocorticoid regulated kinase-1 (SGK1). Activation of SGK1 in the heart causes a marked increase in both the peak and late sodium currents leading to prolongation of the action potential duration and an increased propensity to arrhythmia. Here we show that SGK1 directly regulates NaV1.5 channel function, and genetic inhibition of SGK1 in a zebrafish model of inherited long QT syndrome rescues the long QT phenotype. Using computer-aided drug discovery coupled with in vitro kinase assays, we identified a novel class of SGK1 inhibitors. Our lead SGK1 inhibitor (5377051) selectively inhibits SGK1 in cultured cardiomyocytes, and inhibits phosphorylation of an SGK1-specific target as well as proliferation in the prostate cancer cell line, LNCaP. Finally, 5377051 can reverse SGK1's effects on NaV1.5 and shorten the action potential duration in induced pluripotent stem cell (iPSC)-derived cardiomyocytes from a patient with a gain-of-function mutation in Nav 1.5 (Long QT3 syndrome). Our data suggests that SGK1 inhibitors warrant further investigation in the treatment of cardiac arrhythmias.
Conflict of interest statement
Drs Das, Milan and Rosenzweig have formed a company to develop SGK1 inhibitors for treatment of inherited arrhythmias. They have not received any compensation or research funding from the company. No other authors have any competing financial interests in this project.
Figures
Similar articles
-
Pathological role of serum- and glucocorticoid-regulated kinase 1 in adverse ventricular remodeling.Circulation. 2012 Oct 30;126(18):2208-19. doi: 10.1161/CIRCULATIONAHA.112.115592. Epub 2012 Sep 26. Circulation. 2012. PMID: 23019294 Free PMC article.
-
Serum and Glucocorticoid Regulated Kinase 1 in Sodium Homeostasis.Int J Mol Sci. 2016 Aug 10;17(8):1307. doi: 10.3390/ijms17081307. Int J Mol Sci. 2016. PMID: 27517916 Free PMC article. Review.
-
Protein Phosphatase 2A Regulates Cardiac Na+ Channels.Circ Res. 2019 Mar;124(5):737-746. doi: 10.1161/CIRCRESAHA.118.314350. Circ Res. 2019. PMID: 30602331 Free PMC article.
-
Changes in cardiac Nav1.5 expression, function, and acetylation by pan-histone deacetylase inhibitors.Am J Physiol Heart Circ Physiol. 2016 Nov 1;311(5):H1139-H1149. doi: 10.1152/ajpheart.00156.2016. Epub 2016 Sep 16. Am J Physiol Heart Circ Physiol. 2016. PMID: 27638876 Free PMC article.
-
Therapeutic potential of serum and glucocorticoid inducible kinase inhibition.Expert Opin Investig Drugs. 2013 Jun;22(6):701-14. doi: 10.1517/13543784.2013.778971. Epub 2013 Mar 19. Expert Opin Investig Drugs. 2013. PMID: 23506284 Review.
Cited by
-
Pharmacological suppression of Nedd4-2 rescues the reduction of Kv11.1 channels in pathological cardiac hypertrophy.Front Pharmacol. 2022 Aug 17;13:942769. doi: 10.3389/fphar.2022.942769. eCollection 2022. Front Pharmacol. 2022. PMID: 36059970 Free PMC article.
-
SGK1 inhibition attenuated the action potential duration in patient- and genotype-specific re-engineered heart cells with congenital long QT syndrome.Heart Rhythm O2. 2023 Feb 16;4(4):268-274. doi: 10.1016/j.hroo.2023.02.003. eCollection 2023 Apr. Heart Rhythm O2. 2023. PMID: 37124559 Free PMC article.
-
Serum and Glucocorticoid-Inducible Kinase 1 (SGK1) in NSCLC Therapy.Pharmaceuticals (Basel). 2020 Nov 22;13(11):413. doi: 10.3390/ph13110413. Pharmaceuticals (Basel). 2020. PMID: 33266470 Free PMC article. Review.
-
Serum- and glucocorticoid-inducible kinase 1 and the response to cell stress.Cell Stress. 2018 Dec 2;3(1):1-8. doi: 10.15698/cst2019.01.170. Cell Stress. 2018. PMID: 31225494 Free PMC article. Review.
-
SGK1 induces vascular smooth muscle cell calcification through NF-κB signaling.J Clin Invest. 2018 Jul 2;128(7):3024-3040. doi: 10.1172/JCI96477. Epub 2018 Jun 11. J Clin Invest. 2018. PMID: 29889103 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

