The Role of Angiotensin II in Glomerular Volume Dynamics and Podocyte Calcium Handling
- PMID: 28331185
- PMCID: PMC5428415
- DOI: 10.1038/s41598-017-00406-2
The Role of Angiotensin II in Glomerular Volume Dynamics and Podocyte Calcium Handling
Abstract
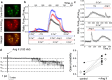
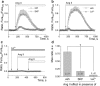
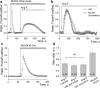
Podocytes are becoming a primary focus of research efforts due to their association with progressive glomeruli damage in disease states. Loss of podocytes can occur as a result of excessive intracellular calcium influx, and we have previously shown that angiotensin II (Ang II) via canonical transient receptor potential 6 (TRPC6) channels caused increased intracellular Ca2+ flux in podocytes. We showed here with patch-clamp electrophysiology that Ang II activates TRPC channels; then using confocal calcium imaging we demonstrated that Ang II-dependent stimulation of Ca2+ influx in the podocytes is precluded by blocking either AT1 or AT2 receptors (ATRs). Application of Ang(1-7) had no effect on intracellular calcium. Ang II-induced calcium flux was decreased upon inhibition of TRPC channels with SAR7334, SKF 96365, clemizole hydrochloride and La3+, but not ML204. Using a novel 3D whole-glomerulus imaging ex vivo assay, we revealed the involvement of both ATRs in controlling glomerular permeability; additionally, using specific inhibitors and activators of TRPC6, we showed that these channels are implicated in the regulation of glomerular volume dynamics. Therefore, we provide evidence demonstrating the critical role of Ang II/TRPC6 axis in the control of glomeruli function, which is likely important for the development of glomerular diseases.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





Similar articles
-
Angiotensin II has acute effects on TRPC6 channels in podocytes of freshly isolated glomeruli.Kidney Int. 2014 Sep;86(3):506-14. doi: 10.1038/ki.2014.71. Epub 2014 Mar 19. Kidney Int. 2014. PMID: 24646854 Free PMC article.
-
Podocyte injury in diabetic nephropathy: implications of angiotensin II-dependent activation of TRPC channels.Sci Rep. 2015 Dec 10;5:17637. doi: 10.1038/srep17637. Sci Rep. 2015. PMID: 26656101 Free PMC article.
-
Angiotensin II induces calcium-mediated autophagy in podocytes through enhancing reactive oxygen species levels.Chem Biol Interact. 2017 Nov 1;277:110-118. doi: 10.1016/j.cbi.2017.09.010. Epub 2017 Sep 12. Chem Biol Interact. 2017. PMID: 28916337
-
TRPC6 channel as an emerging determinant of the podocyte injury susceptibility in kidney diseases.Am J Physiol Renal Physiol. 2015 Sep 1;309(5):F393-7. doi: 10.1152/ajprenal.00186.2015. Epub 2015 Jun 17. Am J Physiol Renal Physiol. 2015. PMID: 26084930 Free PMC article. Review.
-
Balancing calcium signals through TRPC5 and TRPC6 in podocytes.J Am Soc Nephrol. 2011 Nov;22(11):1969-80. doi: 10.1681/ASN.2011040370. Epub 2011 Oct 6. J Am Soc Nephrol. 2011. PMID: 21980113 Free PMC article. Review.
Cited by
-
Cytoskeleton Rearrangements Modulate TRPC6 Channel Activity in Podocytes.Int J Mol Sci. 2021 Apr 22;22(9):4396. doi: 10.3390/ijms22094396. Int J Mol Sci. 2021. PMID: 33922367 Free PMC article.
-
Activation of TRPC6 by AngⅡ Induces Podocyte Injury and Participates in Proteinuria of Nephrotic Syndrome.Front Pharmacol. 2022 Aug 3;13:915153. doi: 10.3389/fphar.2022.915153. eCollection 2022. Front Pharmacol. 2022. PMID: 35991898 Free PMC article.
-
Injured Podocytes Are Sensitized to Angiotensin II-Induced Calcium Signaling.J Am Soc Nephrol. 2020 Mar;31(3):532-542. doi: 10.1681/ASN.2019020109. Epub 2020 Jan 10. J Am Soc Nephrol. 2020. PMID: 31924670 Free PMC article.
-
Role of opioid signaling in kidney damage during the development of salt-induced hypertension.Life Sci Alliance. 2020 Oct 12;3(12):e202000853. doi: 10.26508/lsa.202000853. Print 2020 Dec. Life Sci Alliance. 2020. PMID: 33046522 Free PMC article.
-
Multitarget molecule, PTUPB, to treat diabetic nephropathy in rats.Br J Pharmacol. 2021 Nov;178(22):4468-4484. doi: 10.1111/bph.15623. Epub 2021 Aug 12. Br J Pharmacol. 2021. PMID: 34255857 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

