Canonical Wnt Signaling Ameliorates Aging of Intestinal Stem Cells
- PMID: 28297666
- PMCID: PMC5987258
- DOI: 10.1016/j.celrep.2017.02.056
Canonical Wnt Signaling Ameliorates Aging of Intestinal Stem Cells
Abstract
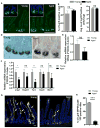
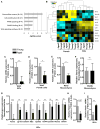
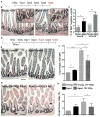
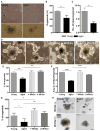
Although intestinal homeostasis is maintained by intestinal stem cells (ISCs), regeneration is impaired upon aging. Here, we first uncover changes in intestinal architecture, cell number, and cell composition upon aging. Second, we identify a decline in the regenerative capacity of ISCs upon aging because of a decline in canonical Wnt signaling in ISCs. Changes in expression of Wnts are found in stem cells themselves and in their niche, including Paneth cells and mesenchyme. Third, reactivating canonical Wnt signaling enhances the function of both murine and human ISCs and, thus, ameliorates aging-associated phenotypes of ISCs in an organoid assay. Our data demonstrate a role for impaired Wnt signaling in physiological aging of ISCs and further identify potential therapeutic avenues to improve ISC regenerative potential upon aging.
Keywords: Wnt signaling; aging; intestinal stem cells; regeneration.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration.Nature. 2015 Dec 24;528(7583):560-564. doi: 10.1038/nature16460. Epub 2015 Dec 9. Nature. 2015. PMID: 26649819 Free PMC article.
-
Regulation of the Paneth cell niche by exogenous L-arginine couples the intestinal stem cell function.FASEB J. 2020 Aug;34(8):10299-10315. doi: 10.1096/fj.201902573RR. Epub 2020 Jun 17. FASEB J. 2020. PMID: 32725957
-
Analysis of Aged Dysfunctional Intestinal Stem Cells.Methods Mol Biol. 2020;2171:41-52. doi: 10.1007/978-1-0716-0747-3_3. Methods Mol Biol. 2020. PMID: 32705634
-
A contemporary snapshot of intestinal stem cells and their regulation.Differentiation. 2019 Jul-Aug;108:3-7. doi: 10.1016/j.diff.2019.01.004. Epub 2019 Jan 25. Differentiation. 2019. PMID: 30711339 Review.
-
Small intestinal stem cells.Curr Opin Gastroenterol. 2013 Mar;29(2):140-5. doi: 10.1097/MOG.0b013e32835cf253. Curr Opin Gastroenterol. 2013. PMID: 23380573 Review.
Cited by
-
Lower human defensin 5 in elderly people compared to middle-aged is associated with differences in the intestinal microbiota composition: the DOSANCO Health Study.Geroscience. 2022 Apr;44(2):997-1009. doi: 10.1007/s11357-021-00398-y. Epub 2021 Jun 8. Geroscience. 2022. PMID: 34105106 Free PMC article.
-
Human ESC-sEVs alleviate age-related bone loss by rejuvenating senescent bone marrow-derived mesenchymal stem cells.J Extracell Vesicles. 2020 Aug 10;9(1):1800971. doi: 10.1080/20013078.2020.1800971. J Extracell Vesicles. 2020. PMID: 32944188 Free PMC article.
-
Age-related changes in intestinal immunity and the microbiome.J Leukoc Biol. 2021 Jun;109(6):1045-1061. doi: 10.1002/JLB.3RI0620-405RR. Epub 2020 Oct 5. J Leukoc Biol. 2021. PMID: 33020981 Free PMC article. Review.
-
Unraveling the journey of cancer stem cells from origin to metastasis.Biochim Biophys Acta Rev Cancer. 2019 Jan;1871(1):50-63. doi: 10.1016/j.bbcan.2018.10.006. Epub 2018 Nov 9. Biochim Biophys Acta Rev Cancer. 2019. PMID: 30419314 Free PMC article. Review.
-
Intestinal stem cell aging at single-cell resolution: Transcriptional perturbations alter cell developmental trajectory reversed by gerotherapeutics.Aging Cell. 2023 May;22(5):e13802. doi: 10.1111/acel.13802. Epub 2023 Mar 2. Aging Cell. 2023. PMID: 36864750 Free PMC article.
References
-
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. - PubMed
-
- Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

