The Transient Receptor Potential Melastatin 7 Channel Regulates Pancreatic Cancer Cell Invasion through the Hsp90α/uPA/MMP2 pathway
- PMID: 28284058
- PMCID: PMC5345960
- DOI: 10.1016/j.neo.2017.01.004
The Transient Receptor Potential Melastatin 7 Channel Regulates Pancreatic Cancer Cell Invasion through the Hsp90α/uPA/MMP2 pathway
Abstract
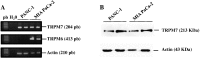
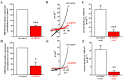
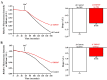
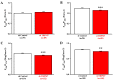
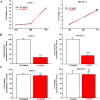
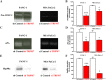
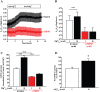
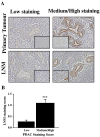
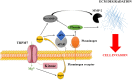
Pancreatic ductal adenocarcinoma (PDAC) is an aggressive malignancy with a very poor prognosis. There is an urgent need to better understand the molecular mechanisms that regulate PDAC cell aggressiveness. The transient receptor potential melastatin 7 (TRPM7) is a nonselective cationic channel that mainly conducts Ca2+ and Mg2+. TRPM7 is overexpressed in numerous malignancies including PDAC. In the present study, we used the PANC-1 and MIA PaCa-2 cell lines to specifically assess the role of TRPM7 in cell invasion and matrix metalloproteinase secretion. We show that TRPM7 regulates Mg2+ homeostasis and constitutive cation entry in both PDAC cell lines. Moreover, cell invasion is strongly reduced by TRPM7 silencing without affecting the cell viability. Conditioned media were further studied, by gel zymography, to detect matrix metalloproteinase (MMP) secretion in PDAC cells. Our results show that MMP-2, urokinase plasminogen activator (uPA), and heat-shock protein 90α (Hsp90α) secretions are significantly decreased in TRPM7-deficient PDAC cells. Moreover, TRPM7 expression in human PDAC lymph node metastasis is correlated to the channel expression in primary tumor. Taken together, our results show that TRPM7 is involved in PDAC cell invasion through regulation of Hsp90α/uPA/MMP-2 proteolytic axis, confirming that this channel could be a promising biomarker and possibly a target for PDAC metastasis therapy.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
TRPM7 overexpression enhances the cancer stem cell-like and metastatic phenotypes of lung cancer through modulation of the Hsp90α/uPA/MMP2 signaling pathway.BMC Cancer. 2018 Nov 26;18(1):1167. doi: 10.1186/s12885-018-5050-x. BMC Cancer. 2018. PMID: 30477473 Free PMC article.
-
Transient receptor potential melastatin-related 7 channel is overexpressed in human pancreatic ductal adenocarcinomas and regulates human pancreatic cancer cell migration.Int J Cancer. 2012 Sep 15;131(6):E851-61. doi: 10.1002/ijc.27487. Epub 2012 Apr 17. Int J Cancer. 2012. PMID: 22323115
-
Recent Advances in Oncogenic Roles of the TRPM7 Chanzyme.Curr Med Chem. 2016;23(36):4092-4107. doi: 10.2174/0929867323666160907162002. Curr Med Chem. 2016. PMID: 27604090
-
TRPM7 and its role in neurodegenerative diseases.Channels (Austin). 2015;9(5):253-61. doi: 10.1080/19336950.2015.1075675. Epub 2015 Jul 28. Channels (Austin). 2015. PMID: 26218331 Free PMC article. Review.
-
Oncogenic role and therapeutic target of transient receptor potential melastatin 7 channel in malignancy.Expert Opin Ther Targets. 2014 Oct;18(10):1177-96. doi: 10.1517/14728222.2014.940894. Epub 2014 Jul 29. Expert Opin Ther Targets. 2014. PMID: 25069584 Review.
Cited by
-
Wip1 is associated with tumorigenity and metastasis through MMP-2 in human intrahepatic cholangiocarcinoma.Oncotarget. 2017 May 23;8(34):56672-56683. doi: 10.18632/oncotarget.18074. eCollection 2017 Aug 22. Oncotarget. 2017. PMID: 28915621 Free PMC article.
-
TRPM Family Channels in Cancer.Pharmaceuticals (Basel). 2018 Jun 7;11(2):58. doi: 10.3390/ph11020058. Pharmaceuticals (Basel). 2018. PMID: 29875336 Free PMC article. Review.
-
Isoalantolactone inhibits pancreatic cancer proliferation by regulation of PI3K and Wnt signal pathway.PLoS One. 2021 Mar 4;16(3):e0247752. doi: 10.1371/journal.pone.0247752. eCollection 2021. PLoS One. 2021. PMID: 33661942 Free PMC article.
-
Circulating uPA as a potential prognostic biomarker for resectable esophageal squamous cell carcinoma.Medicine (Baltimore). 2019 Mar;98(9):e14717. doi: 10.1097/MD.0000000000014717. Medicine (Baltimore). 2019. PMID: 30817615 Free PMC article.
-
Expression Profiling Identified TRPM7 and HER2 as Potential Targets for the Combined Treatment of Cancer Cells.Cells. 2024 Oct 31;13(21):1801. doi: 10.3390/cells13211801. Cells. 2024. PMID: 39513908 Free PMC article.
References
-
- Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol. 2010;7:163–172. - PubMed
-
- Prevarskaya N, Skryma R, Shuba Y. Calcium in tumour metastasis: new roles for known actors. Nat Rev Cancer. 2011;11:609–618. - PubMed
-
- Stock C, Schwab A. Ion channels and transporters in metastasis. Biochim Biophys Acta. 2015;1848:2638–2646. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

