Hepatitis C virus NS3 protein enhances hepatocellular carcinoma cell invasion by promoting PPM1A ubiquitination and degradation
- PMID: 28283039
- PMCID: PMC5345236
- DOI: 10.1186/s13046-017-0510-8
Hepatitis C virus NS3 protein enhances hepatocellular carcinoma cell invasion by promoting PPM1A ubiquitination and degradation
Abstract
Background: Growing evidence suggests that hepatitis C virus (HCV) contributes to hepatocellular carcinoma (HCC) by directly modulating oncogenic signaling pathways. Protein phosphatase magnesium-dependent 1A (PPM1A) has recently emerged as an important tumor suppressor as it can block a range of tumor-centric signaling pathways through protein dephosphorylation. However, the role and regulatory mechanisms of PPM1A in HCV-infected cells have not been reported.
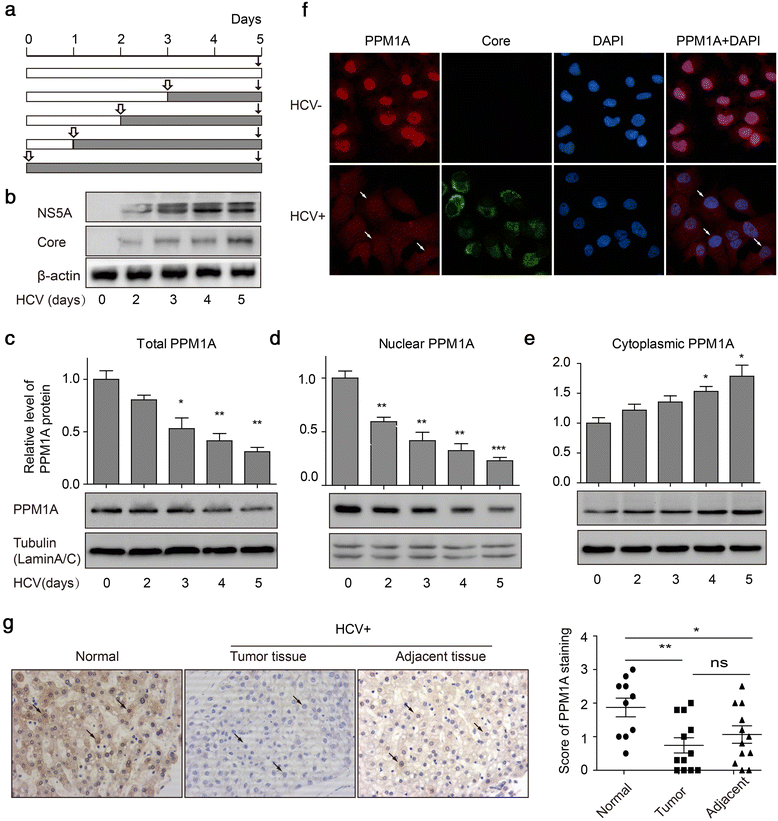
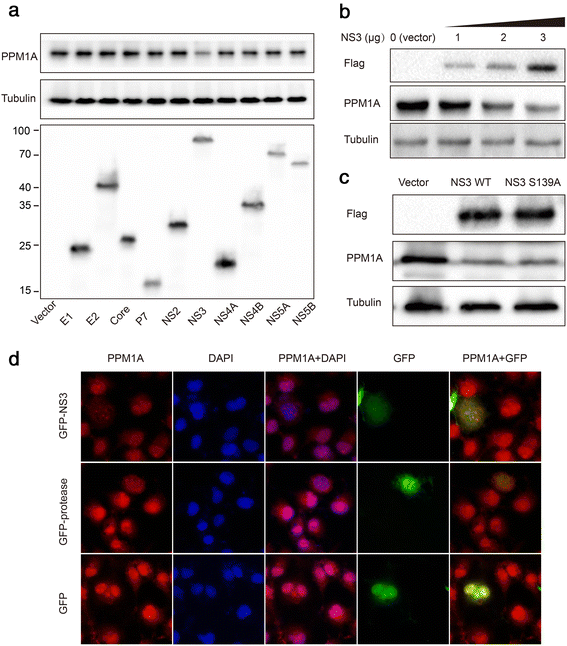
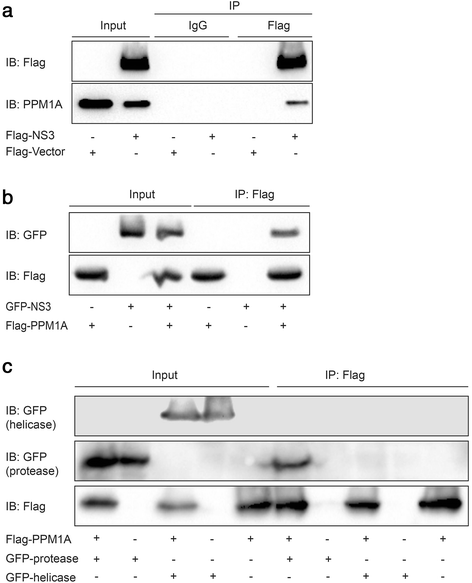
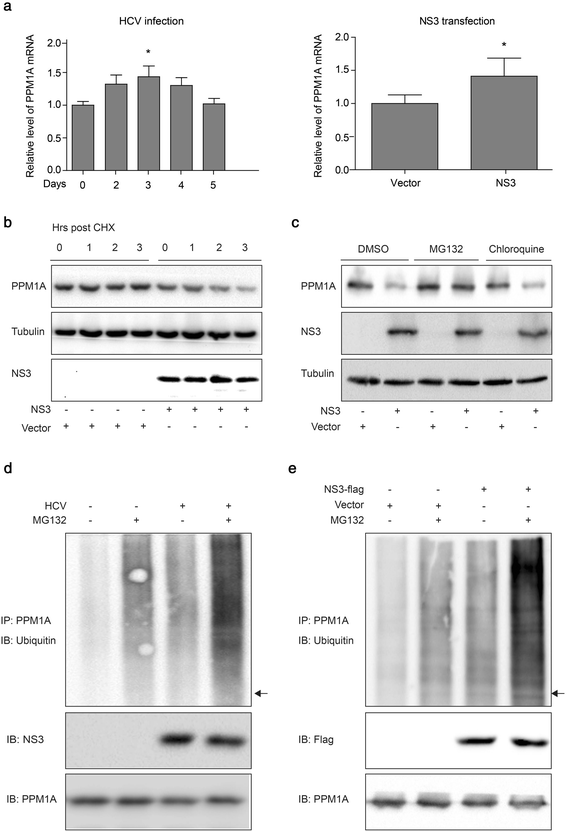
Methods: Total, cytoplasmic, and nuclear PPM1A protein after HCV infection or overexpression of HCV nonstructural protein 3 (NS3) were detected by western blotting. The expression of PPM1A in normal liver and HCV-related HCC tissues was quantified by immunohistochemistry. The effects of HCV infection and NS3 expression on the PPM1A protein level were systematically analyzed, and the ubiquitination level of PPM1A was determined by precipitation with anti-PPM1A and immunoblotting with either anti-ubiquitin or anti-PPM1A antibody. Finally, the roles of NS3 and PPM1A in hepatoma cell migration and invasion were assessed by wound healing and transwell assays, respectively.
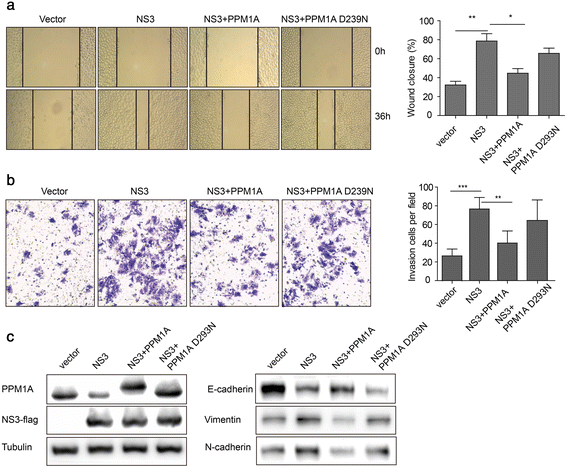
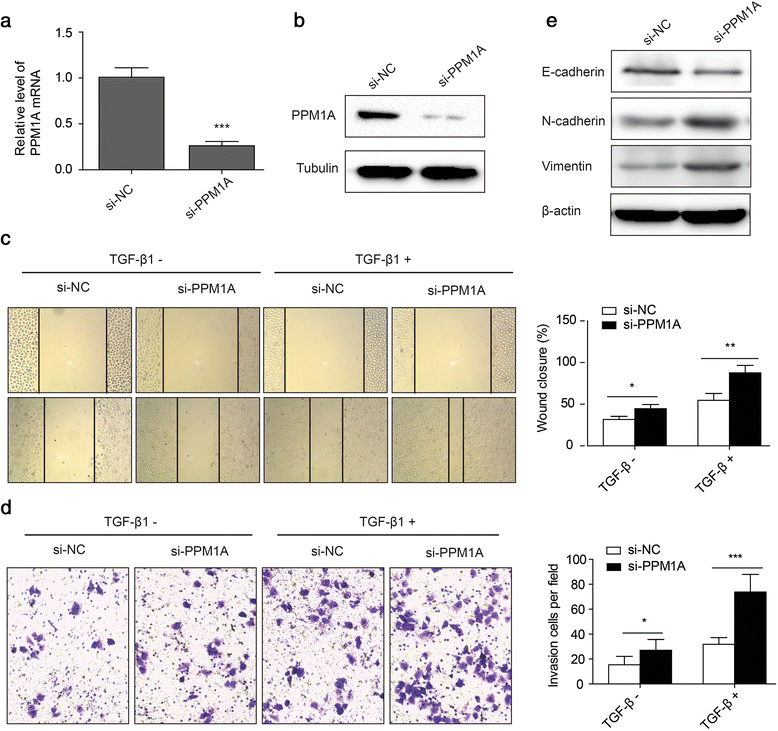
Results: HCV infection and replication decreased PPM1A abundance, mediated by NS3, in hepatoma cells. Compared to normal liver tissues, the expression of PPM1A was significantly decreased in the HCC tumor tissues and adjacent non-tumor tissues. NS3 directly interacted with PPM1A to promote PPM1A ubiquitination and degradation, which was dependent on its protease domain. Blockade of PPM1A through small interfering RNA significantly promoted HCC cell migration, invasion, and epithelial mesenchymal transition (EMT), which were further intensified by TGF-β1 stimulation, in vitro. Furthermore, restoration of PPM1A abrogated the NS3-mediated promotion of HCC migration and invasion to a great extent, which was dependent on its protein phosphatase function.
Conclusions: Our findings demonstrate that the HCV protein NS3 can downregulate PPM1A by promoting its ubiquitination and proteasomal degradation, which might contribute to the migration and invasion of hepatoma cells and may represent a new strategy of HCV in carcinogenesis.
Keywords: Cancer cell invasion; Hepatitis C virus; Hepatocellular carcinoma; PPM1A; Ubiquitination and degradation.
Figures
Similar articles
-
TRIM52 up-regulation in hepatocellular carcinoma cells promotes proliferation, migration and invasion through the ubiquitination of PPM1A.J Exp Clin Cancer Res. 2018 Jun 13;37(1):116. doi: 10.1186/s13046-018-0780-9. J Exp Clin Cancer Res. 2018. PMID: 29898761 Free PMC article.
-
Hepatitis B virus X protein amplifies TGF-β promotion on HCC motility through down-regulating PPM1a.Oncotarget. 2016 May 31;7(22):33125-35. doi: 10.18632/oncotarget.8884. Oncotarget. 2016. PMID: 27121309 Free PMC article.
-
Hepatitis C virus NS3 protein enhances cancer cell invasion by activating matrix metalloproteinase-9 and cyclooxygenase-2 through ERK/p38/NF-κB signal cascade.Cancer Lett. 2015 Jan 28;356(2 Pt B):470-8. doi: 10.1016/j.canlet.2014.09.027. Epub 2014 Oct 8. Cancer Lett. 2015. PMID: 25305454
-
Viral hepatitis and liver cancer: the case of hepatitis C.Oncogene. 2006 Jun 26;25(27):3834-47. doi: 10.1038/sj.onc.1209562. Oncogene. 2006. PMID: 16799625 Review.
-
Hepatitis C-associated liver carcinogenesis: role of PML nuclear bodies.World J Gastroenterol. 2014 Sep 21;20(35):12367-71. doi: 10.3748/wjg.v20.i35.12367. World J Gastroenterol. 2014. PMID: 25253937 Free PMC article. Review.
Cited by
-
Hepatocellular Carcinoma: Prevention, Diagnosis, and Treatment.Med Princ Pract. 2024;33(5):414-423. doi: 10.1159/000539349. Epub 2024 May 21. Med Princ Pract. 2024. PMID: 38772352 Free PMC article. Review.
-
Human endogenous retrovirus W env increases nitric oxide production and enhances the migration ability of microglia by regulating the expression of inducible nitric oxide synthase.Virol Sin. 2017 Jun;32(3):216-225. doi: 10.1007/s12250-017-3997-4. Epub 2017 Jun 23. Virol Sin. 2017. PMID: 28656540 Free PMC article.
-
High expression of ubiquitin-conjugating enzyme E2A predicts poor prognosis in hepatocellular carcinoma.Oncol Lett. 2018 May;15(5):7362-7368. doi: 10.3892/ol.2018.8189. Epub 2018 Mar 7. Oncol Lett. 2018. PMID: 29725449 Free PMC article.
-
Contextual Regulation of TGF-β Signaling in Liver Cancer.Cells. 2019 Oct 11;8(10):1235. doi: 10.3390/cells8101235. Cells. 2019. PMID: 31614569 Free PMC article. Review.
-
A comprehensive overview of PPM1A: From structure to disease.Exp Biol Med (Maywood). 2022 Mar;247(6):453-461. doi: 10.1177/15353702211061883. Epub 2021 Dec 3. Exp Biol Med (Maywood). 2022. PMID: 34861123 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

