Cell-specific deletion of C1qa identifies microglia as the dominant source of C1q in mouse brain
- PMID: 28264694
- PMCID: PMC5340039
- DOI: 10.1186/s12974-017-0814-9
Cell-specific deletion of C1qa identifies microglia as the dominant source of C1q in mouse brain
Abstract
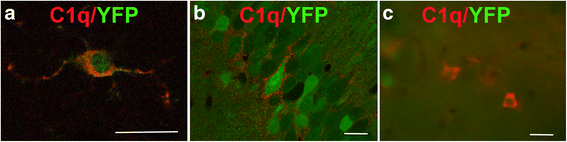
Background: The complement cascade not only provides protection from infection but can also mediate destructive inflammation. Complement is also involved in elimination of neuronal synapses which is essential for proper development, but can be detrimental during aging and disease. C1q, required for several of these complement-mediated activities, is present in the neuropil, microglia, and a subset of interneurons in the brain.
Methods: To identify the source(s) of C1q in the brain, the C1qa gene was selectively inactivated in the microglia or Thy-1+ neurons in both wild type mice and a mouse model of Alzheimer's disease (AD), and C1q synthesis assessed by immunohistochemistry, QPCR, and western blot analysis.
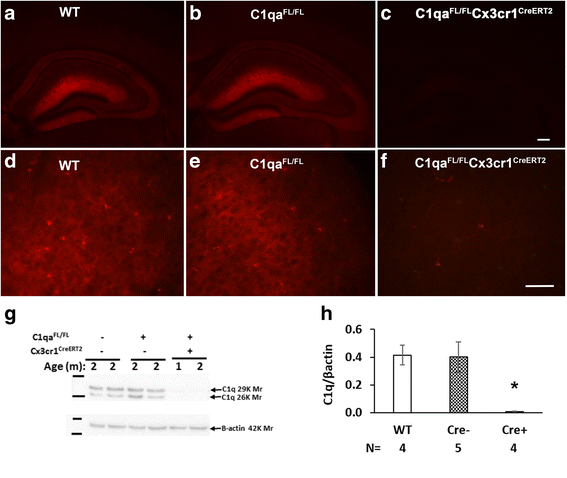
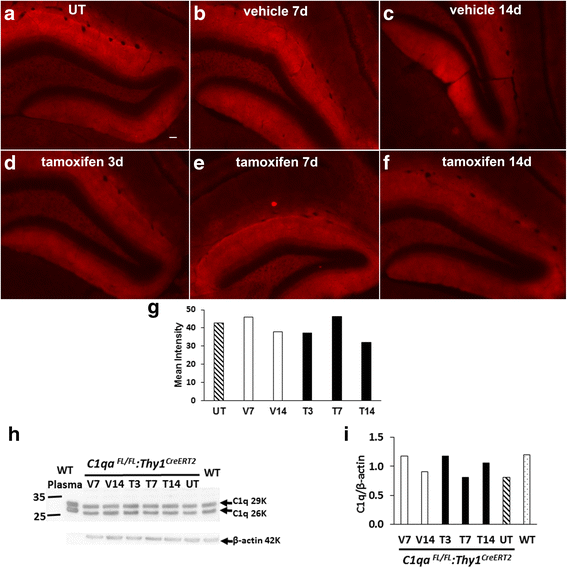
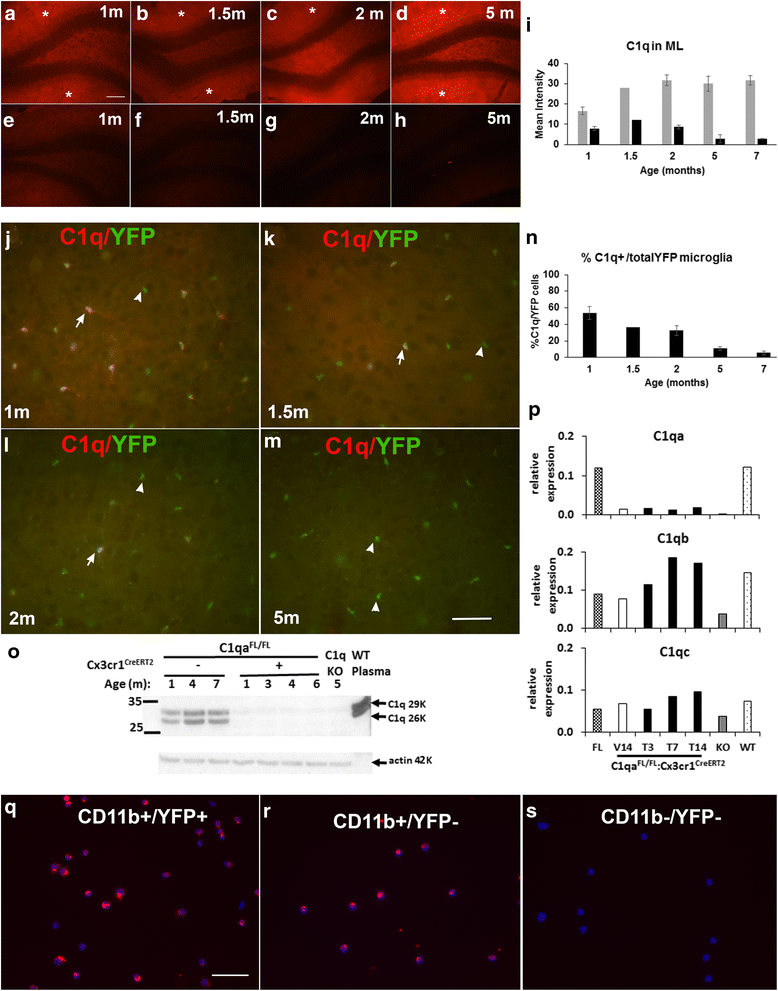
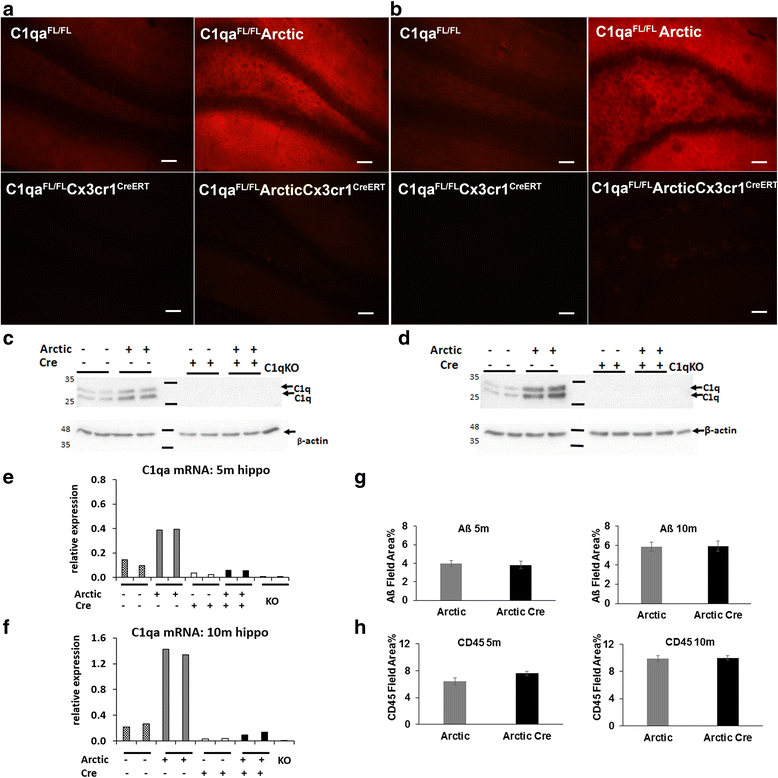
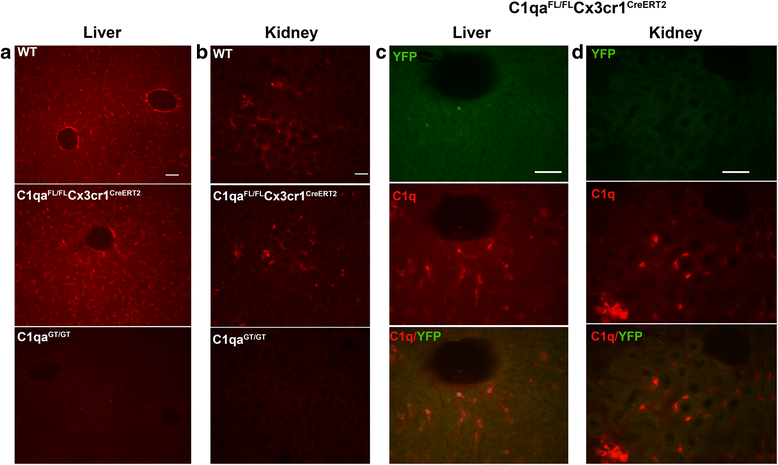
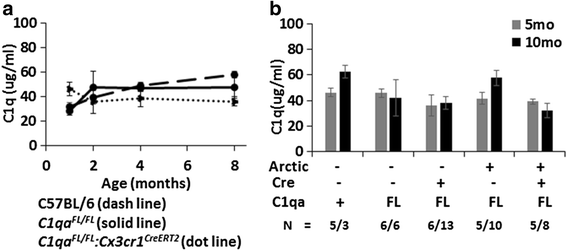
Results: While C1q expression in the brain was unaffected after inactivation of C1qa in Thy-1+ neurons, the brains of C1qa FL/FL :Cx3cr1 CreERT2 mice in which C1qa was ablated in microglia were devoid of C1q with the exception of limited C1q in subsets of interneurons. Surprisingly, this loss of C1q occurred even in the absence of tamoxifen by 1 month of age, demonstrating that Cre activity is tamoxifen-independent in microglia in Cx3cr1 CreERT2/WganJ mice. C1q expression in C1qa FL/FL : Cx3cr1 CreERT2/WganJ mice continued to decline and remained almost completely absent through aging and in AD model mice. No difference in C1q was detected in the liver or kidney from C1qa FL/FL : Cx3cr1 CreERT2/WganJ mice relative to controls, and C1qa FL/FL : Cx3cr1 CreERT2/WganJ mice had minimal, if any, reduction in plasma C1q.
Conclusions: Thus, microglia, but not neurons or peripheral sources, are the dominant source of C1q in the brain. While demonstrating that the Cx3cr1 CreERT2/WganJ deleter cannot be used for adult-induced deletion of genes in microglia, the model described here enables further investigation of physiological roles of C1q in the brain and identification of therapeutic targets for the selective control of complement-mediated activities contributing to neurodegenerative disorders.
Keywords: Alzheimer’s; C1q; Complement; Conditional knockout; Expression; Microglia; Mouse model.
Figures
Similar articles
-
Subretinal macrophages produce classical complement activator C1q leading to the progression of focal retinal degeneration.Mol Neurodegener. 2018 Aug 20;13(1):45. doi: 10.1186/s13024-018-0278-0. Mol Neurodegener. 2018. PMID: 30126455 Free PMC article.
-
CX3CR1 in microglia regulates brain amyloid deposition through selective protofibrillar amyloid-β phagocytosis.J Neurosci. 2010 Dec 15;30(50):17091-101. doi: 10.1523/JNEUROSCI.4403-10.2010. J Neurosci. 2010. PMID: 21159979 Free PMC article.
-
Peroxisome Proliferator-Activated Receptor-δ Deficiency in Microglia Results in Exacerbated Axonal Injury and Tissue Loss in Experimental Autoimmune Encephalomyelitis.Front Immunol. 2021 Feb 26;12:570425. doi: 10.3389/fimmu.2021.570425. eCollection 2021. Front Immunol. 2021. PMID: 33732230 Free PMC article.
-
Evidence that immunoglobulin-positive neurons in Alzheimer's disease are dying via the classical antibody-dependent complement pathway.Am J Alzheimers Dis Other Demen. 2005 May-Jun;20(3):144-50. doi: 10.1177/153331750502000303. Am J Alzheimers Dis Other Demen. 2005. PMID: 16003929 Free PMC article. Review.
-
Complement in Human Brain Health: Potential of Dietary Food in Relation to Neurodegenerative Diseases.Foods. 2023 Sep 26;12(19):3580. doi: 10.3390/foods12193580. Foods. 2023. PMID: 37835232 Free PMC article. Review.
Cited by
-
Loss of C1q alters the auditory brainstem response.Front Cell Neurosci. 2024 Oct 2;18:1464670. doi: 10.3389/fncel.2024.1464670. eCollection 2024. Front Cell Neurosci. 2024. PMID: 39416682 Free PMC article.
-
Dysregulated C1q and CD47 in the aging monkey brain: association with myelin damage, microglia reactivity, and cognitive decline.Front Immunol. 2024 Sep 27;15:1426975. doi: 10.3389/fimmu.2024.1426975. eCollection 2024. Front Immunol. 2024. PMID: 39399501 Free PMC article.
-
Microglia in physiological conditions and the importance of understanding their homeostatic functions in the arcuate nucleus.Front Immunol. 2024 Sep 4;15:1392077. doi: 10.3389/fimmu.2024.1392077. eCollection 2024. Front Immunol. 2024. PMID: 39295865 Free PMC article. Review.
-
C5aR1 antagonism suppresses inflammatory glial responses and alters cellular signaling in an Alzheimer's disease mouse model.Nat Commun. 2024 Aug 15;15(1):7028. doi: 10.1038/s41467-024-51163-6. Nat Commun. 2024. PMID: 39147742 Free PMC article.
-
C1qa Muscularis Macrophages Regulate Gastrointestinal Motility Through Close Association With Enteric Neurons.Gastro Hep Adv. 2023 Aug 19;2(8):1028-1031. doi: 10.1016/j.gastha.2023.08.007. eCollection 2023. Gastro Hep Adv. 2023. PMID: 39131550 Free PMC article. Review. No abstract available.
References
-
- Brekke OL, Waage C, Christiansen D, Fure H, Qu H, Lambris JD, Osterud B, Nielsen EW, Mollnes TE. The effects of selective complement and CD14 inhibition on the E. coli-induced tissue factor mRNA upregulation, monocyte tissue factor expression, and tissue factor functional activity in human whole blood. Adv Exp Med Biol. 2013;734:123–136. doi: 10.1007/978-1-4614-4118-2_8. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

