Characterizing the Structure and Oligomerization of Major Royal Jelly Protein 1 (MRJP1) by Mass Spectrometry and Complementary Biophysical Tools
- PMID: 28252287
- PMCID: PMC5450925
- DOI: 10.1021/acs.biochem.7b00020
Characterizing the Structure and Oligomerization of Major Royal Jelly Protein 1 (MRJP1) by Mass Spectrometry and Complementary Biophysical Tools
Abstract
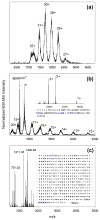
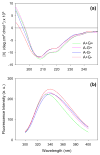
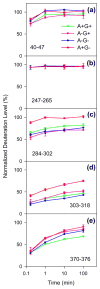
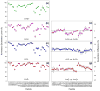
Royal jelly (RJ) triggers the development of female honeybee larvae into queens. This effect has been attributed to the presence of major royal jelly protein 1 (MRJP1) in RJ. MRJP1 isolated from royal jelly is tightly associated with apisimin, a 54-residue α-helical peptide that promotes the noncovalent assembly of MRJP1 into multimers. No high-resolution structural data are available for these complexes, and their binding stoichiometry remains uncertain. We examined MRJP1/apisimin using a range of biophysical techniques. We also investigated the behavior of deglycosylated samples, as well as samples with reduced apisimin content. Our mass spectrometry (MS) data demonstrate that the native complexes predominantly exist in a (MRJP14 apisimin4) stoichiometry. Hydrogen/deuterium exchange MS reveals that MRJP1 within these complexes is extensively disordered in the range of residues 20-265. Marginally stable secondary structure (likely antiparallel β-sheet) exists around residues 266-432. These weakly structured regions interchange with conformers that are extensively unfolded, giving rise to bimodal (EX1) isotope distributions. We propose that the native complexes have a "dimer of dimers" quaternary structure in which MRJP1 chains are bridged by apisimin. Specifically, our data suggest that apisimin acts as a linker that forms hydrophobic contacts involving the MRJP1 segment 316VLFFGLV322. Deglycosylation produces large soluble aggregates, highlighting the role of glycans as aggregation inhibitors. Samples with reduced apisimin content form dimeric complexes with a (MRJP12 apisimin1) stoichiometry. The information uncovered in this work will help pave the way toward a better understanding of the unique physiological role played by MRJP1 during queen differentiation.
Figures

Similar articles
-
Structure of native glycolipoprotein filaments in honeybee royal jelly.Nat Commun. 2020 Dec 8;11(1):6267. doi: 10.1038/s41467-020-20135-x. Nat Commun. 2020. PMID: 33293513 Free PMC article.
-
Architecture of the native major royal jelly protein 1 oligomer.Nat Commun. 2018 Aug 22;9(1):3373. doi: 10.1038/s41467-018-05619-1. Nat Commun. 2018. PMID: 30135511 Free PMC article.
-
Quantification of Major Royal Jelly Protein 1 in Fresh Royal Jelly by Ultraperformance Liquid Chromatography-Tandem Mass Spectrometry.J Agric Food Chem. 2018 Feb 7;66(5):1270-1278. doi: 10.1021/acs.jafc.7b05698. Epub 2018 Jan 30. J Agric Food Chem. 2018. PMID: 29381065
-
Origin and function of the major royal jelly proteins of the honeybee (Apis mellifera) as members of the yellow gene family.Biol Rev Camb Philos Soc. 2014 May;89(2):255-69. doi: 10.1111/brv.12052. Epub 2013 Jul 16. Biol Rev Camb Philos Soc. 2014. PMID: 23855350 Review.
-
New Insights into the Biological and Pharmaceutical Properties of Royal Jelly.Int J Mol Sci. 2020 Jan 8;21(2):382. doi: 10.3390/ijms21020382. Int J Mol Sci. 2020. PMID: 31936187 Free PMC article. Review.
Cited by
-
Antioxidant Potential of Propolis, Bee Pollen, and Royal Jelly: Possible Medical Application.Oxid Med Cell Longev. 2018 May 2;2018:7074209. doi: 10.1155/2018/7074209. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 29854089 Free PMC article. Review.
-
Structure of native glycolipoprotein filaments in honeybee royal jelly.Nat Commun. 2020 Dec 8;11(1):6267. doi: 10.1038/s41467-020-20135-x. Nat Commun. 2020. PMID: 33293513 Free PMC article.
-
Architecture of the native major royal jelly protein 1 oligomer.Nat Commun. 2018 Aug 22;9(1):3373. doi: 10.1038/s41467-018-05619-1. Nat Commun. 2018. PMID: 30135511 Free PMC article.
-
DNA Methylation in Honey Bees and the Unresolved Questions in Insect Methylomics.Adv Exp Med Biol. 2022;1389:159-176. doi: 10.1007/978-3-031-11454-0_7. Adv Exp Med Biol. 2022. PMID: 36350510
-
pH-dependent stability of honey bee (Apis mellifera) major royal jelly proteins.Sci Rep. 2019 Jun 21;9(1):9014. doi: 10.1038/s41598-019-45460-0. Sci Rep. 2019. PMID: 31227768 Free PMC article.
References
-
- Haydak MH. Honey Bee Nutrition. Annu Rev Entomol. 1970;15:143–156.
-
- Patel NG, Haydak MH, Gochnauer TA. Electrophoretic components of the proteins in honeybee larval food. Nature. 1960;186:633–634. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

