Ultrastructural Characterization of Zika Virus Replication Factories
- PMID: 28249158
- PMCID: PMC5340982
- DOI: 10.1016/j.celrep.2017.02.014
Ultrastructural Characterization of Zika Virus Replication Factories
Abstract
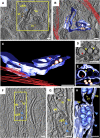
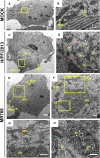
A global concern has emerged with the pandemic spread of Zika virus (ZIKV) infections that can cause severe neurological symptoms in adults and newborns. ZIKV is a positive-strand RNA virus replicating in virus-induced membranous replication factories (RFs). Here we used various imaging techniques to investigate the ultrastructural details of ZIKV RFs and their relationship with host cell organelles. Analyses of human hepatic cells and neural progenitor cells infected with ZIKV revealed endoplasmic reticulum (ER) membrane invaginations containing pore-like openings toward the cytosol, reminiscent to RFs in Dengue virus-infected cells. Both the MR766 African strain and the H/PF/2013 Asian strain, the latter linked to neurological diseases, induce RFs of similar architecture. Importantly, ZIKV infection causes a drastic reorganization of microtubules and intermediate filaments forming cage-like structures surrounding the viral RF. Consistently, ZIKV replication is suppressed by cytoskeleton-targeting drugs. Thus, ZIKV RFs are tightly linked to rearrangements of the host cell cytoskeleton.
Keywords: Zika virus; electron microscopy; electron tomography; flavivirus; human neural progenitor cells; intermediate filaments; live-cell imaging; microtubules; replication factories; replication organelles.
Copyright © 2017 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Zika virus induced cellular remodelling.Cell Microbiol. 2017 Aug;19(8):10.1111/cmi.12740. doi: 10.1111/cmi.12740. Epub 2017 Apr 18. Cell Microbiol. 2017. PMID: 28318141 Free PMC article.
-
Host cytoskeletal vimentin serves as a structural organizer and an RNA-binding protein regulator to facilitate Zika viral replication.Proc Natl Acad Sci U S A. 2022 Feb 22;119(8):e2113909119. doi: 10.1073/pnas.2113909119. Proc Natl Acad Sci U S A. 2022. PMID: 35193960 Free PMC article.
-
Zika Virus Infection Induces DNA Damage Response in Human Neural Progenitors That Enhances Viral Replication.J Virol. 2019 Sep 30;93(20):e00638-19. doi: 10.1128/JVI.00638-19. Print 2019 Oct 15. J Virol. 2019. PMID: 31375586 Free PMC article.
-
Host-Virus Interaction of ZIKA Virus in Modulating Disease Pathogenesis.J Neuroimmune Pharmacol. 2017 Jun;12(2):219-232. doi: 10.1007/s11481-017-9736-7. Epub 2017 Mar 27. J Neuroimmune Pharmacol. 2017. PMID: 28349242 Free PMC article. Review.
-
Endoplasmic reticulum: a focal point of Zika virus infection.J Biomed Sci. 2020 Jan 20;27(1):27. doi: 10.1186/s12929-020-0618-6. J Biomed Sci. 2020. PMID: 31959174 Free PMC article. Review.
Cited by
-
Nuclear membrane protein SUN2 promotes replication of flaviviruses through modulating cytoskeleton reorganization mediated by NS1.Nat Commun. 2024 Jan 5;15(1):296. doi: 10.1038/s41467-023-44580-6. Nat Commun. 2024. PMID: 38177122 Free PMC article.
-
Zika Virus-Mediated Death of Hippocampal Neurons Is Independent From Maturation State.Front Cell Neurosci. 2019 Aug 27;13:389. doi: 10.3389/fncel.2019.00389. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31551711 Free PMC article.
-
Let's Get Physical: Flavivirus-Host Protein-Protein Interactions in Replication and Pathogenesis.Front Microbiol. 2022 Mar 3;13:847588. doi: 10.3389/fmicb.2022.847588. eCollection 2022. Front Microbiol. 2022. PMID: 35308381 Free PMC article. Review.
-
Role of Host Cell Secretory Machinery in Zika Virus Life Cycle.Viruses. 2018 Oct 15;10(10):559. doi: 10.3390/v10100559. Viruses. 2018. PMID: 30326556 Free PMC article. Review.
-
Manipulation of Host Microtubule Networks by Viral Microtubule-Associated Proteins.Viruses. 2022 May 6;14(5):979. doi: 10.3390/v14050979. Viruses. 2022. PMID: 35632720 Free PMC article. Review.
References
-
- Acosta E.G., Kumar A., Bartenschlager R. Revisiting dengue virus-host cell interaction: new insights into molecular and cellular virology. Adv. Virus Res. 2014;88:1–109. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

