Defining stem cell dynamics and migration during wound healing in mouse skin epidermis
- PMID: 28248284
- PMCID: PMC5339881
- DOI: 10.1038/ncomms14684
Defining stem cell dynamics and migration during wound healing in mouse skin epidermis
Abstract
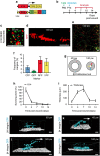
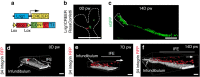
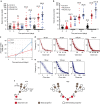
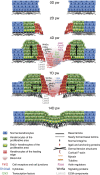
Wound healing is essential to repair the skin after injury. In the epidermis, distinct stem cells (SCs) populations contribute to wound healing. However, how SCs balance proliferation, differentiation and migration to repair a wound remains poorly understood. Here, we show the cellular and molecular mechanisms that regulate wound healing in mouse tail epidermis. Using a combination of proliferation kinetics experiments and molecular profiling, we identify the gene signatures associated with proliferation, differentiation and migration in different regions surrounding the wound. Functional experiments show that SC proliferation, migration and differentiation can be uncoupled during wound healing. Lineage tracing and quantitative clonal analysis reveal that, following wounding, progenitors divide more rapidly, but conserve their homoeostatic mode of division, leading to their rapid depletion, whereas SCs become active, giving rise to new progenitors that expand and repair the wound. These results have important implications for tissue regeneration, acute and chronic wound disorders.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

Similar articles
-
Single-Cell Transcriptomics of Traced Epidermal and Hair Follicle Stem Cells Reveals Rapid Adaptations during Wound Healing.Cell Rep. 2018 Oct 16;25(3):585-597.e7. doi: 10.1016/j.celrep.2018.09.059. Cell Rep. 2018. PMID: 30332640
-
Modulatory role of sensory innervation on hair follicle stem cell progeny during wound healing of the rat skin.PLoS One. 2012;7(5):e36421. doi: 10.1371/journal.pone.0036421. Epub 2012 May 4. PLoS One. 2012. PMID: 22574159 Free PMC article.
-
Expansion of epidermal progenitors with high p63 phosphorylation during wound healing of mouse epidermis.Exp Dermatol. 2013 May;22(5):374-6. doi: 10.1111/exd.12139. Exp Dermatol. 2013. PMID: 23614751 Free PMC article.
-
Vitamin D and calcium regulation of epidermal wound healing.J Steroid Biochem Mol Biol. 2016 Nov;164:379-385. doi: 10.1016/j.jsbmb.2015.08.011. Epub 2015 Aug 14. J Steroid Biochem Mol Biol. 2016. PMID: 26282157 Free PMC article. Review.
-
Epithelial stem cells and implications for wound repair.Semin Cell Dev Biol. 2012 Dec;23(9):946-53. doi: 10.1016/j.semcdb.2012.10.001. Epub 2012 Oct 17. Semin Cell Dev Biol. 2012. PMID: 23085626 Free PMC article. Review.
Cited by
-
Deep learning for rapid analysis of cell divisions in vivo during epithelial morphogenesis and repair.Elife. 2024 Sep 23;12:RP87949. doi: 10.7554/eLife.87949. Elife. 2024. PMID: 39312468 Free PMC article.
-
Macrophages in Skin Wounds: Functions and Therapeutic Potential.Biomolecules. 2022 Nov 8;12(11):1659. doi: 10.3390/biom12111659. Biomolecules. 2022. PMID: 36359009 Free PMC article. Review.
-
Single-cell RNA sequencing identifies a migratory keratinocyte subpopulation expressing THBS1 in epidermal wound healing.iScience. 2022 Mar 21;25(4):104130. doi: 10.1016/j.isci.2022.104130. eCollection 2022 Apr 15. iScience. 2022. PMID: 35391830 Free PMC article.
-
Stem cell dynamics, migration and plasticity during wound healing.Nat Cell Biol. 2019 Jan;21(1):18-24. doi: 10.1038/s41556-018-0237-6. Epub 2019 Jan 2. Nat Cell Biol. 2019. PMID: 30602767 Free PMC article. Review.
-
Making sense of fragmentation and merging in lineage tracing experiments.Front Cell Dev Biol. 2022 Dec 14;10:1054476. doi: 10.3389/fcell.2022.1054476. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36589749 Free PMC article. Review.
References
-
- Arwert E. N., Hoste E. & Watt F. M. Epithelial stem cells, wound healing and cancer. Nat. Rev. Cancer. 12, 170–180 (2012). - PubMed
-
- Gurtner G. C., Werner S., Barrandon Y. & Longaker M. T. Wound repair and regeneration. Nature 453, 314–321 (2008). - PubMed
-
- Coulombe P. A. Wound epithelialization: accelerating the pace of discovery. J. Invest. Dermatol. 121, 219–230 (2003). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

