Optical control of cell signaling by single-chain photoswitchable kinases
- PMID: 28232577
- PMCID: PMC5589340
- DOI: 10.1126/science.aah3605
Optical control of cell signaling by single-chain photoswitchable kinases
Abstract
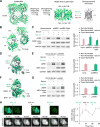
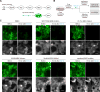
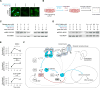
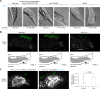
Protein kinases transduce signals to regulate a wide array of cellular functions in eukaryotes. A generalizable method for optical control of kinases would enable fine spatiotemporal interrogation or manipulation of these various functions. We report the design and application of single-chain cofactor-free kinases with photoswitchable activity. We engineered a dimeric protein, pdDronpa, that dissociates in cyan light and reassociates in violet light. Attaching two pdDronpa domains at rationally selected locations in the kinase domain, we created the photoswitchable kinases psRaf1, psMEK1, psMEK2, and psCDK5. Using these photoswitchable kinases, we established an all-optical cell-based assay for screening inhibitors, uncovered a direct and rapid inhibitory feedback loop from ERK to MEK1, and mediated developmental changes and synaptic vesicle transport in vivo using light.
Copyright © 2017, American Association for the Advancement of Science.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Similar articles
-
Rational Design and Synthesis of Diverse Pyrimidine Molecules Bearing Sulfonamide Moiety as Novel ERK Inhibitors.Int J Mol Sci. 2019 Nov 8;20(22):5592. doi: 10.3390/ijms20225592. Int J Mol Sci. 2019. PMID: 31717402 Free PMC article.
-
MEK-ERK inhibition corrects the defect in VLDL assembly in HepG2 cells: potential role of ERK in VLDL-ApoB100 particle assembly.Arterioscler Thromb Vasc Biol. 2007 Jan;27(1):211-8. doi: 10.1161/01.ATV.0000249861.80471.96. Epub 2006 Oct 12. Arterioscler Thromb Vasc Biol. 2007. PMID: 17038630
-
Characterization of a novel mitogen-activated protein kinase kinase 1/2 inhibitor with a unique mechanism of action for cancer therapy.Cancer Res. 2009 Mar 1;69(5):1924-32. doi: 10.1158/0008-5472.CAN-08-2627. Epub 2009 Feb 24. Cancer Res. 2009. PMID: 19244124
-
Allosteric modulators of MEK1: drug design and discovery.Chem Biol Drug Des. 2016 Oct;88(4):485-97. doi: 10.1111/cbdd.12780. Epub 2016 Jun 1. Chem Biol Drug Des. 2016. PMID: 27115708 Review.
-
MEK1/2 Inhibitors: Molecular Activity and Resistance Mechanisms.Semin Oncol. 2015 Dec;42(6):849-62. doi: 10.1053/j.seminoncol.2015.09.023. Epub 2015 Sep 24. Semin Oncol. 2015. PMID: 26615130 Free PMC article. Review.
Cited by
-
NF-κB signaling dynamics is controlled by a dose-sensing autoregulatory loop.Sci Signal. 2019 Apr 30;12(579):eaau3568. doi: 10.1126/scisignal.aau3568. Sci Signal. 2019. PMID: 31040261 Free PMC article.
-
Cellular Complexity in MAPK Signaling in Plants: Questions and Emerging Tools to Answer Them.Front Plant Sci. 2018 Nov 27;9:1674. doi: 10.3389/fpls.2018.01674. eCollection 2018. Front Plant Sci. 2018. PMID: 30538711 Free PMC article. Review.
-
Optically inducible membrane recruitment and signaling systems.Curr Opin Struct Biol. 2019 Aug;57:84-92. doi: 10.1016/j.sbi.2019.01.017. Epub 2019 Mar 16. Curr Opin Struct Biol. 2019. PMID: 30884362 Free PMC article. Review.
-
Towards translational optogenetics.Nat Biomed Eng. 2023 Apr;7(4):349-369. doi: 10.1038/s41551-021-00829-3. Epub 2022 Jan 13. Nat Biomed Eng. 2023. PMID: 35027688 Review.
-
Light-switchable transcription factors obtained by direct screening in mammalian cells.Nat Commun. 2023 Jun 2;14(1):3185. doi: 10.1038/s41467-023-38993-6. Nat Commun. 2023. PMID: 37268649 Free PMC article.
References
-
- Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. - PubMed
-
- Bergeron JJ, Di Guglielmo GM, Dahan S, Dominguez M, Posner BI. Spatial and Temporal Regulation of Receptor Tyrosine Kinase Activation and Intracellular Signal Transduction. Annu Rev Biochem. 2016 - PubMed
-
- Shimizu-Sato S, Huq E, Tepperman JM, Quail PH. A light-switchable gene promoter system. Nat Biotechnol. 2002;20:1041–1044. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

