Expansion of myeloid-derived suppressor cells with aging in the bone marrow of mice through a NF-κB-dependent mechanism
- PMID: 28229533
- PMCID: PMC5418207
- DOI: 10.1111/acel.12571
Expansion of myeloid-derived suppressor cells with aging in the bone marrow of mice through a NF-κB-dependent mechanism
Abstract
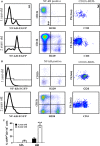
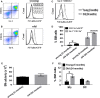
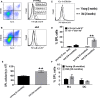
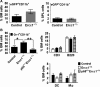
With aging, there is progressive loss of tissue homeostasis and functional reserve, leading to an impaired response to stress and an increased risk of morbidity and mortality. A key mediator of the cellular response to damage and stress is the transcription factor NF-κB. We demonstrated previously that NF-κB transcriptional activity is upregulated in tissues from both natural aged mice and in a mouse model of a human progeroid syndrome caused by defective repair of DNA damage (ERCC1-deficient mice). We also demonstrated that genetic reduction in the level of the NF-κB subunit p65(RelA) in the Ercc1-/∆ progeroid mouse model of accelerated aging delayed the onset of age-related pathology including muscle wasting, osteoporosis, and intervertebral disk degeneration. Here, we report that the largest fraction of NF-κB -expressing cells in the bone marrow (BM) of aged (>2 year old) mice (C57BL/6-NF-κBEGFP reporter mice) are Gr-1+ CD11b+ myeloid-derived suppressor cells (MDSCs). There was a significant increase in the overall percentage of MDSC present in the BM of aged animals compared with young, a trend also observed in the spleen. However, the function of these cells appears not to be compromised in aged mice. A similar increase of MDSC was observed in BM of progeroid Ercc1-/∆ and BubR1H/H mice. The increase in MDSC in Ercc1-/∆ mice was abrogated by heterozygosity in the p65/RelA subunit of NF-κB. These results suggest that NF-κB activation with aging, at least in part, drives an increase in the percentage of MDSCs, a cell type able to suppress immune cell responses.
Keywords: NF-κB; myeloid-derived suppressor cell; senescence.
© 2017 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Figures

Similar articles
-
ISSLS prize winner: inhibition of NF-κB activity ameliorates age-associated disc degeneration in a mouse model of accelerated aging.Spine (Phila Pa 1976). 2012 Oct 1;37(21):1819-25. doi: 10.1097/BRS.0b013e31824ee8f7. Spine (Phila Pa 1976). 2012. PMID: 22343279 Free PMC article.
-
NF-κB inhibition delays DNA damage-induced senescence and aging in mice.J Clin Invest. 2012 Jul;122(7):2601-12. doi: 10.1172/JCI45785. Epub 2012 Jun 18. J Clin Invest. 2012. PMID: 22706308 Free PMC article.
-
DNA damage drives accelerated bone aging via an NF-κB-dependent mechanism.J Bone Miner Res. 2013 May;28(5):1214-28. doi: 10.1002/jbmr.1851. J Bone Miner Res. 2013. PMID: 23281008 Free PMC article.
-
Myeloid-derived suppressor cells (MDSC): an important partner in cellular/tissue senescence.Biogerontology. 2018 Oct;19(5):325-339. doi: 10.1007/s10522-018-9762-8. Epub 2018 Jun 29. Biogerontology. 2018. PMID: 29959657 Review.
-
The role of myeloid-derived suppressor cells (MDSC) in the inflammaging process.Ageing Res Rev. 2018 Dec;48:1-10. doi: 10.1016/j.arr.2018.09.001. Epub 2018 Sep 21. Ageing Res Rev. 2018. PMID: 30248408 Review.
Cited by
-
Myeloid-Derived Suppressor Cells at the Intersection of Inflammaging and Bone Fragility.Immunol Invest. 2018 Nov;47(8):844-854. doi: 10.1080/08820139.2018.1552360. Immunol Invest. 2018. PMID: 31282803 Free PMC article. Review.
-
Aging Mouse Models Reveal Complex Tumor-Microenvironment Interactions in Cancer Progression.Front Cell Dev Biol. 2018 Mar 29;6:35. doi: 10.3389/fcell.2018.00035. eCollection 2018. Front Cell Dev Biol. 2018. PMID: 29651417 Free PMC article. Review.
-
Age-mediated gut microbiota dysbiosis promotes the loss of dendritic cells tolerance.Aging Cell. 2023 Jun;22(6):e13838. doi: 10.1111/acel.13838. Epub 2023 May 9. Aging Cell. 2023. PMID: 37161603 Free PMC article.
-
The Roles of Myeloid Cells in Aging-related Liver Diseases.Int J Biol Sci. 2023 Mar 5;19(5):1564-1578. doi: 10.7150/ijbs.82352. eCollection 2023. Int J Biol Sci. 2023. PMID: 37056921 Free PMC article. Review.
-
Chronic Critical Illness and the Persistent Inflammation, Immunosuppression, and Catabolism Syndrome.Front Immunol. 2018 Jul 2;9:1511. doi: 10.3389/fimmu.2018.01511. eCollection 2018. Front Immunol. 2018. PMID: 30013565 Free PMC article. Review.
References
-
- Baker DJ, Jeganathan KB, Cameron JD, Thompson M, Juneja S, Kopecka A, Kumar R, Jenkins RB, de Groen PC, Roche P, van Deursen JM (2004) BubR1 insufficiency causes early onset of aging‐associated phenotypes and infertility in mice. Nat. Genet. 36, 744–749. - PubMed
-
- Bartlett DB, Firth CM, Phillips AC, Moss P, Baylis D, Syddall H, Sayer AA, Cooper C, Lord JM (2012) The age‐related increase in low‐grade systemic inflammation (Inflammaging) is not driven by cytomegalovirus infection. Aging Cell 11, 912–915. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

