Long-term treatment with ruxolitinib for patients with myelofibrosis: 5-year update from the randomized, double-blind, placebo-controlled, phase 3 COMFORT-I trial
- PMID: 28228106
- PMCID: PMC5322633
- DOI: 10.1186/s13045-017-0417-z
Long-term treatment with ruxolitinib for patients with myelofibrosis: 5-year update from the randomized, double-blind, placebo-controlled, phase 3 COMFORT-I trial
Abstract
Background: The randomized, double-blind, placebo-controlled, phase 3 COMFORT-I trial evaluated the JAK1/JAK2 inhibitor ruxolitinib in patients with intermediate-2/high-risk myelofibrosis. The primary and planned 3-year analyses of COMFORT-I data demonstrated that ruxolitinib-the first myelofibrosis-approved therapy-reduced splenomegaly and prolonged overall survival versus placebo. Here, we present the final 5-year results.
Methods: Patients managed in Australia, Canada, and the USA were randomized centrally (interactive voice response system) 1:1 to oral ruxolitinib twice daily (15 or 20 mg per baseline platelet counts) or placebo. Investigators and patients were blinded to treatment. The secondary endpoints evaluated in this analysis were durability of a ≥35% reduction from baseline in spleen volume (spleen response) and overall survival, evaluated in the intent-to-treat population. Safety was evaluated in patients who received study treatment.
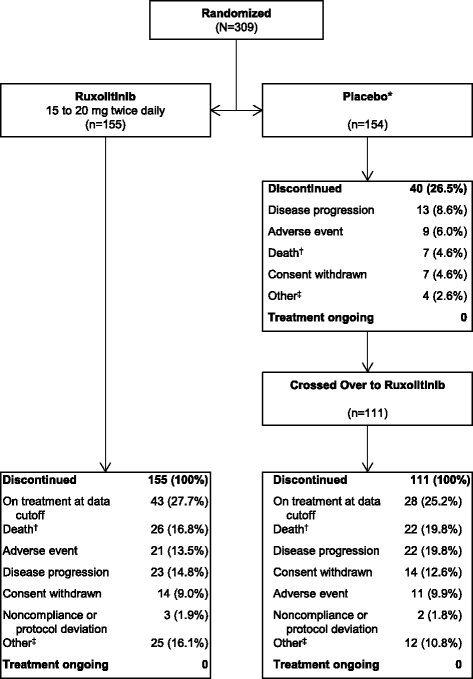
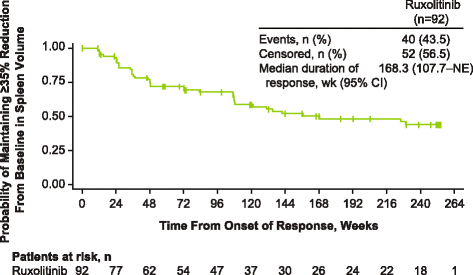
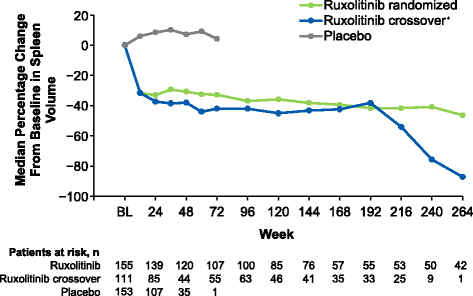
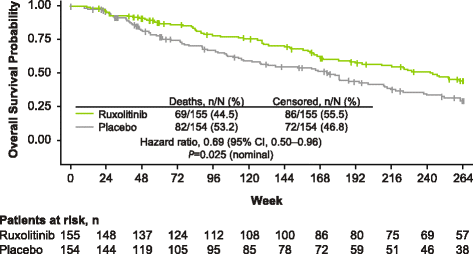
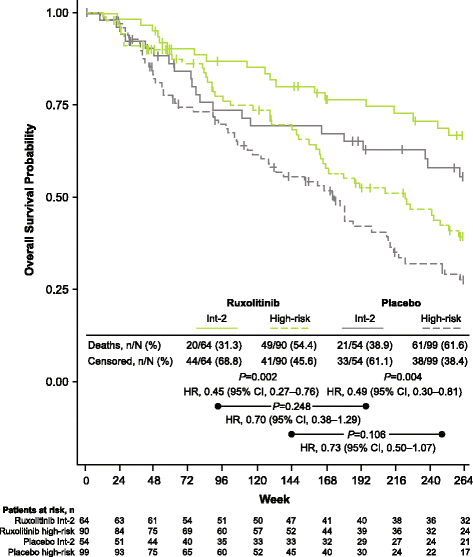
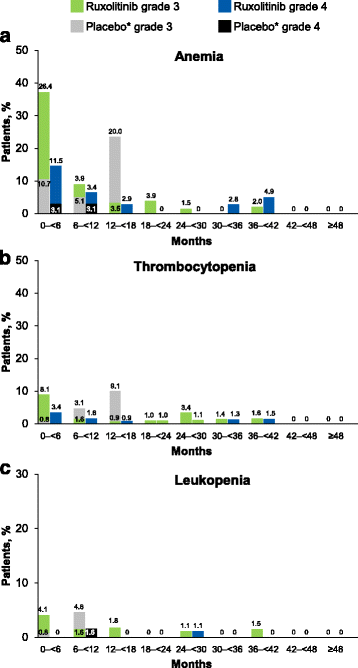
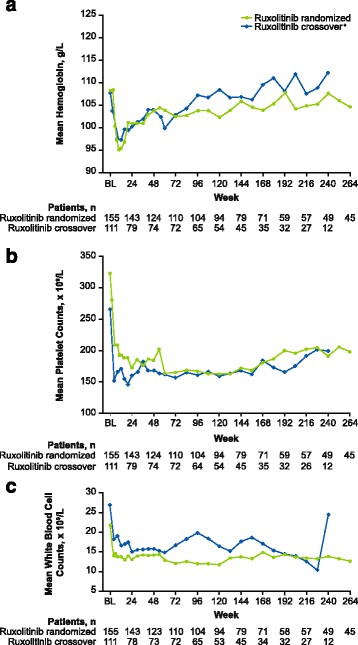
Results: Patients were randomized (September 2009-April 2010) to ruxolitinib (n = 155) or placebo (n = 154). At termination, 27.7% of ruxolitinib-randomized patients and 25.2% (28/111) who crossed over from placebo were on treatment; no patients remained on placebo. Patients randomized to ruxolitinib had a median spleen response duration of 168.3 weeks and prolonged median overall survival versus placebo (ruxolitinib group, not reached; placebo group, 200 weeks; HR, 0.69; 95% CI, 0.50-0.96; P = 0.025) despite the crossover to ruxolitinib. The ruxolitinib safety profile remained consistent with previous analyses. The most common new-onset all-grade nonhematologic adverse events starting <12 versus ≥48 months after ruxolitinib initiation were fatigue (29.0 vs 33.3%) and diarrhea (27.8 vs 14.6%). New-onset grade 3 or 4 anemia and thrombocytopenia both primarily occurred within the first 6 months, with no cases after 42 months. The most common treatment-emergent adverse event-related deaths in the ruxolitinib-randomized group were sepsis (2.6%), disease progression (1.9%), and pneumonia (1.9%).
Conclusion: The final COMFORT-I results continue to support ruxolitinib as an effective treatment for patients with intermediate-2/high-risk MF.
Trial registration: ClinicalTrials.gov, NCT00952289.
Keywords: JAK; Janus kinase; Myelofibrosis.
Figures
Similar articles
-
A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis.N Engl J Med. 2012 Mar 1;366(9):799-807. doi: 10.1056/NEJMoa1110557. N Engl J Med. 2012. PMID: 22375971 Free PMC article. Clinical Trial.
-
Long-term survival in patients treated with ruxolitinib for myelofibrosis: COMFORT-I and -II pooled analyses.J Hematol Oncol. 2017 Sep 29;10(1):156. doi: 10.1186/s13045-017-0527-7. J Hematol Oncol. 2017. PMID: 28962635 Free PMC article. Clinical Trial.
-
Efficacy, safety, and survival with ruxolitinib in patients with myelofibrosis: results of a median 3-year follow-up of COMFORT-I.Haematologica. 2015 Apr;100(4):479-88. doi: 10.3324/haematol.2014.115840. Epub 2015 Jan 23. Haematologica. 2015. PMID: 25616577 Free PMC article.
-
Ruxolitinib for myelofibrosis--an update of its clinical effects.Clin Lymphoma Myeloma Leuk. 2013 Dec;13(6):638-45. doi: 10.1016/j.clml.2013.09.006. Epub 2013 Oct 2. Clin Lymphoma Myeloma Leuk. 2013. PMID: 24238036 Free PMC article. Review.
-
Janus kinase-1 and Janus kinase-2 inhibitors for treating myelofibrosis.Cochrane Database Syst Rev. 2015 Apr 10;2015(4):CD010298. doi: 10.1002/14651858.CD010298.pub2. Cochrane Database Syst Rev. 2015. PMID: 25860512 Free PMC article. Review.
Cited by
-
Monocyte-regulated interleukin 12 production drives clearance of Staphylococcus aureus.PLoS Pathog. 2024 Oct 17;20(10):e1012648. doi: 10.1371/journal.ppat.1012648. eCollection 2024 Oct. PLoS Pathog. 2024. PMID: 39418302 Free PMC article.
-
Improved Outcomes in Myelofibrosis after Allogeneic Stem-Cell Transplantation in the Era of Ruxolitinib Pretreatment and Intensified Conditioning Regimen-Single-Center Analysis.Cancers (Basel). 2024 Sep 25;16(19):3257. doi: 10.3390/cancers16193257. Cancers (Basel). 2024. PMID: 39409879 Free PMC article.
-
Comparative efficacy and hematologic safety of different dosages of JAK inhibitors in the treatment of myelofibrosis: a network meta-analysis.Front Oncol. 2024 Aug 30;14:1403967. doi: 10.3389/fonc.2024.1403967. eCollection 2024. Front Oncol. 2024. PMID: 39281381 Free PMC article.
-
Molecular biomarkers of leukemia: convergence-based drug resistance mechanisms in chronic myeloid leukemia and myeloproliferative neoplasms.Front Pharmacol. 2024 Jul 22;15:1422565. doi: 10.3389/fphar.2024.1422565. eCollection 2024. Front Pharmacol. 2024. PMID: 39104388 Free PMC article. Review.
-
Type II mode of JAK2 inhibition and destabilization are potential therapeutic approaches against the ruxolitinib resistance driven myeloproliferative neoplasms.Front Oncol. 2024 Jul 18;14:1430833. doi: 10.3389/fonc.2024.1430833. eCollection 2024. Front Oncol. 2024. PMID: 39091915 Free PMC article.
References
-
- Hultcrantz M, Kristinsson SY, Andersson TM, Landgren O, Eloranta S, Derolf AR, et al. Patterns of survival among patients with myeloproliferative neoplasms diagnosed in Sweden from 1973 to 2008: a population-based study. J Clin Oncol. 2012;30:2995–3001. doi: 10.1200/JCO.2012.42.1925. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

