Therapeutic antibody targeting of indoleamine-2,3-dioxygenase (IDO2) inhibits autoimmune arthritis
- PMID: 28223071
- PMCID: PMC5466478
- DOI: 10.1016/j.clim.2017.01.016
Therapeutic antibody targeting of indoleamine-2,3-dioxygenase (IDO2) inhibits autoimmune arthritis
Abstract
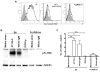
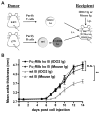
Rheumatoid arthritis (RA) is a debilitating inflammatory autoimmune disease with no known cure. Recently, we identified the immunomodulatory enzyme indoleamine-2,3-dioxygenase 2 (IDO2) as an essential mediator of autoreactive B and T cell responses driving RA. However, therapeutically targeting IDO2 has been challenging given the lack of small molecules that specifically inhibit IDO2 without also affecting the closely related IDO1. In this study, we develop a novel monoclonal antibody (mAb)-based approach to therapeutically target IDO2. Treatment with IDO2-specific mAb alleviated arthritis in two independent preclinical arthritis models, reducing autoreactive T and B cell activation and recapitulating the strong anti-arthritic effect of genetic IDO2 deficiency. Mechanistic investigations identified FcγRIIb as necessary for mAb internalization, allowing targeting of an intracellular antigen traditionally considered inaccessible to mAb therapy. Taken together, our results offer preclinical proof of concept for antibody-mediated targeting of IDO2 as a new therapeutic strategy to treat RA and other autoantibody-mediated diseases.
Keywords: Antibody therapy; B cells; Experimental arthritis; IDO; Murine model.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures
Comment in
-
Experimental arthritis: Do you want to treat arthritis? IDO2!Nat Rev Rheumatol. 2017 Apr;13(4):196-197. doi: 10.1038/nrrheum.2017.33. Epub 2017 Mar 9. Nat Rev Rheumatol. 2017. PMID: 28275264 No abstract available.
Similar articles
-
Impact of IDO1 and IDO2 on the B Cell Immune Response.Front Immunol. 2022 Apr 13;13:886225. doi: 10.3389/fimmu.2022.886225. eCollection 2022. Front Immunol. 2022. PMID: 35493480 Free PMC article. Review.
-
IDO2 is a critical mediator of autoantibody production and inflammatory pathogenesis in a mouse model of autoimmune arthritis.J Immunol. 2014 Mar 1;192(5):2082-2090. doi: 10.4049/jimmunol.1303012. Epub 2014 Jan 31. J Immunol. 2014. PMID: 24489090 Free PMC article.
-
Differential Roles of IDO1 and IDO2 in T and B Cell Inflammatory Immune Responses.Front Immunol. 2020 Aug 18;11:1861. doi: 10.3389/fimmu.2020.01861. eCollection 2020. Front Immunol. 2020. PMID: 32973768 Free PMC article.
-
IDO2 Modulates T Cell-Dependent Autoimmune Responses through a B Cell-Intrinsic Mechanism.J Immunol. 2016 Jun 1;196(11):4487-97. doi: 10.4049/jimmunol.1600141. Epub 2016 Apr 25. J Immunol. 2016. PMID: 27183624 Free PMC article.
-
IDO2: A Pathogenic Mediator of Inflammatory Autoimmunity.Clin Med Insights Pathol. 2016 Nov 21;9(Suppl 1):21-28. doi: 10.4137/CPath.S39930. eCollection 2016. Clin Med Insights Pathol. 2016. PMID: 27891058 Free PMC article. Review.
Cited by
-
Causal relationship between serum metabolites and juvenile idiopathic arthritis: a mendelian randomization study.Pediatr Rheumatol Online J. 2024 May 9;22(1):51. doi: 10.1186/s12969-024-00986-0. Pediatr Rheumatol Online J. 2024. PMID: 38724970 Free PMC article.
-
A large meta-analysis identifies genes associated with anterior uveitis.Nat Commun. 2023 Nov 11;14(1):7300. doi: 10.1038/s41467-023-43036-1. Nat Commun. 2023. PMID: 37949852 Free PMC article.
-
Impact of IDO1 and IDO2 on the B Cell Immune Response.Front Immunol. 2022 Apr 13;13:886225. doi: 10.3389/fimmu.2022.886225. eCollection 2022. Front Immunol. 2022. PMID: 35493480 Free PMC article. Review.
-
Metabolites as drivers and targets in rheumatoid arthritis.Clin Exp Immunol. 2022 Jun 11;208(2):167-180. doi: 10.1093/cei/uxab021. Clin Exp Immunol. 2022. PMID: 35020864 Free PMC article.
-
The Immunomodulatory Enzyme IDO2 Mediates Autoimmune Arthritis through a Nonenzymatic Mechanism.J Immunol. 2022 Feb 1;208(3):571-581. doi: 10.4049/jimmunol.2100705. Epub 2021 Dec 29. J Immunol. 2022. PMID: 34965962 Free PMC article.
References
-
- Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58:15–25. - PubMed
-
- Gibofsky A. Epidemiology, pathophysiology, and diagnosis of rheumatoid arthritis: A Synopsis. Am J Manag Care. 2014;20:s128–45. - PubMed
-
- Singh JA, Furst DE, Bharat A, Curtis JR, Kavanaugh AF, Kremer JM, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64:625–39. - PMC - PubMed
-
- Alghasham A, Rasheed Z. Therapeutic targets for rheumatoid arthritis: Progress and promises. Autoimmunity. 2014 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

