Systemic delivery of factor IX messenger RNA for protein replacement therapy
- PMID: 28202722
- PMCID: PMC5347596
- DOI: 10.1073/pnas.1619653114
Systemic delivery of factor IX messenger RNA for protein replacement therapy
Abstract
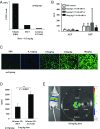
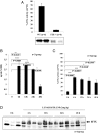
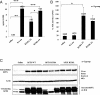
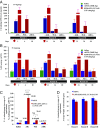
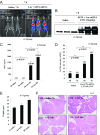
Safe and efficient delivery of messenger RNAs for protein replacement therapies offers great promise but remains challenging. In this report, we demonstrate systemic, in vivo, nonviral mRNA delivery through lipid nanoparticles (LNPs) to treat a Factor IX (FIX)-deficient mouse model of hemophilia B. Delivery of human FIX (hFIX) mRNA encapsulated in our LUNAR LNPs results in a rapid pulse of FIX protein (within 4-6 h) that remains stable for up to 4-6 d and is therapeutically effective, like the recombinant human factor IX protein (rhFIX) that is the current standard of care. Extensive cytokine and liver enzyme profiling showed that repeated administration of the mRNA-LUNAR complex does not cause any adverse innate or adaptive immune responses in immune-competent, hemophilic mice. The levels of hFIX protein that were produced also remained consistent during repeated administrations. These results suggest that delivery of long mRNAs is a viable therapeutic alternative for many clotting disorders and for other hepatic diseases where recombinant proteins may be unaffordable or unsuitable.
Keywords: hemophilia B therapy; hepatic diseases; lipid nanoparticles; nonviral mRNA delivery; systemic delivery.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Employing a gain-of-function factor IX variant R338L to advance the efficacy and safety of hemophilia B human gene therapy: preclinical evaluation supporting an ongoing adeno-associated virus clinical trial.Hum Gene Ther. 2015 Feb;26(2):69-81. doi: 10.1089/hum.2014.106. Epub 2015 Jan 21. Hum Gene Ther. 2015. PMID: 25419787 Free PMC article.
-
Intraarticular factor IX protein or gene replacement protects against development of hemophilic synovitis in the absence of circulating factor IX.Blood. 2008 Dec 1;112(12):4532-41. doi: 10.1182/blood-2008-01-131417. Epub 2008 Aug 20. Blood. 2008. PMID: 18716130 Free PMC article.
-
Therapeutic efficacy in a hemophilia B model using a biosynthetic mRNA liver depot system.Gene Ther. 2016 Oct;23(10):699-707. doi: 10.1038/gt.2016.46. Epub 2016 Jun 30. Gene Ther. 2016. PMID: 27356951 Free PMC article.
-
Gene therapy for hemophilia.Curr Opin Mol Ther. 1999 Aug;1(4):493-9. Curr Opin Mol Ther. 1999. PMID: 11713765 Review.
-
Hemophilia Gene Therapy: Ready for Prime Time?Hum Gene Ther. 2017 Nov;28(11):1013-1023. doi: 10.1089/hum.2017.116. Epub 2017 Aug 3. Hum Gene Ther. 2017. PMID: 28793786 Review.
Cited by
-
Biomedical applications of mRNA nanomedicine.Nano Res. 2018 Oct;11(10):5281-5309. doi: 10.1007/s12274-018-2146-1. Epub 2018 Jul 27. Nano Res. 2018. PMID: 31007865 Free PMC article.
-
Lipid nanoparticle delivers phenylalanine ammonia lyase mRNA to the liver leading to catabolism and clearance of phenylalanine in a phenylketonuria mouse model.Mol Genet Metab Rep. 2022 May 14;32:100882. doi: 10.1016/j.ymgmr.2022.100882. eCollection 2022 Sep. Mol Genet Metab Rep. 2022. PMID: 35600090 Free PMC article.
-
Recent Advancement in mRNA Vaccine Development and Applications.Pharmaceutics. 2023 Jul 18;15(7):1972. doi: 10.3390/pharmaceutics15071972. Pharmaceutics. 2023. PMID: 37514158 Free PMC article. Review.
-
Branched chemically modified poly(A) tails enhance the translation capacity of mRNA.Nat Biotechnol. 2025 Feb;43(2):194-203. doi: 10.1038/s41587-024-02174-7. Epub 2024 Mar 22. Nat Biotechnol. 2025. PMID: 38519719 Free PMC article.
-
Ionizable lipid nanoparticles for in utero mRNA delivery.Sci Adv. 2021 Jan 13;7(3):eaba1028. doi: 10.1126/sciadv.aba1028. Print 2021 Jan. Sci Adv. 2021. PMID: 33523869 Free PMC article.
References
-
- Sahin U, Karikó K, Türeci Ö. mRNA-based therapeutics: Developing a new class of drugs. Nat Rev Drug Discov. 2014;13(10):759–780. - PubMed
-
- Yin H, et al. Non-viral vectors for gene-based therapy. Nat Rev Genet. 2014;15(8):541–555. - PubMed
-
- Jirikowski GF, Sanna PP, Maciejewski-Lenoir D, Bloom FE. Reversal of diabetes insipidus in Brattleboro rats: Intrahypothalamic injection of vasopressin mRNA. Science. 1992;255(5047):996–998. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

