Radiation Resistance in KRAS-Mutated Lung Cancer Is Enabled by Stem-like Properties Mediated by an Osteopontin-EGFR Pathway
- PMID: 28202526
- PMCID: PMC5445902
- DOI: 10.1158/0008-5472.CAN-16-0808
Radiation Resistance in KRAS-Mutated Lung Cancer Is Enabled by Stem-like Properties Mediated by an Osteopontin-EGFR Pathway
Abstract
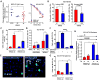
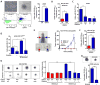
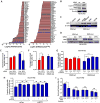
Lung cancers with activating KRAS mutations are characterized by treatment resistance and poor prognosis. In particular, the basis for their resistance to radiation therapy is poorly understood. Here, we describe a radiation resistance phenotype conferred by a stem-like subpopulation characterized by mitosis-like condensed chromatin (MLCC), high CD133 expression, invasive potential, and tumor-initiating properties. Mechanistic investigations defined a pathway involving osteopontin and the EGFR in promoting this phenotype. Osteopontin/EGFR-dependent MLCC protected cells against radiation-induced DNA double-strand breaks and repressed putative negative regulators of stem-like properties, such as CRMP1 and BIM. The MLCC-positive phenotype defined a subset of KRAS-mutated lung cancers that were enriched for co-occurring genomic alterations in TP53 and CDKN2A. Our results illuminate the basis for the radiation resistance of KRAS-mutated lung cancers, with possible implications for prognostic and therapeutic strategies. Cancer Res; 77(8); 2018-28. ©2017 AACR.
©2017 American Association for Cancer Research.
Conflict of interest statement
Figures


Similar articles
-
Tyr1068-phosphorylated epidermal growth factor receptor (EGFR) predicts cancer stem cell targeting by erlotinib in preclinical models of wild-type EGFR lung cancer.Cell Death Dis. 2015 Aug 6;6(8):e1850. doi: 10.1038/cddis.2015.217. Cell Death Dis. 2015. PMID: 26247735 Free PMC article.
-
The EGFR mutation status affects the relative biological effectiveness of carbon-ion beams in non-small cell lung carcinoma cells.Sci Rep. 2015 Jun 11;5:11305. doi: 10.1038/srep11305. Sci Rep. 2015. PMID: 26065573 Free PMC article.
-
PTEN mutations and relationship to EGFR, ERBB2, KRAS, and TP53 mutations in non-small cell lung cancers.Lung Cancer. 2010 Sep;69(3):279-83. doi: 10.1016/j.lungcan.2009.11.012. Epub 2009 Dec 16. Lung Cancer. 2010. PMID: 20018398
-
Targeting metabolic reprogramming in KRAS-driven cancers.Int J Clin Oncol. 2017 Aug;22(4):651-659. doi: 10.1007/s10147-017-1156-4. Epub 2017 Jun 24. Int J Clin Oncol. 2017. PMID: 28647837 Review.
-
Meta-analysis of epidermal growth factor receptor and KRAS gene status between primary and corresponding metastatic tumours of non-small cell lung cancer.Clin Oncol (R Coll Radiol). 2015 Jan;27(1):30-9. doi: 10.1016/j.clon.2014.09.014. Epub 2014 Oct 14. Clin Oncol (R Coll Radiol). 2015. PMID: 25445553 Review.
Cited by
-
A novel PPARɣ ligand, PPZ023, overcomes radioresistance via ER stress and cell death in human non-small-cell lung cancer cells.Exp Mol Med. 2020 Oct;52(10):1730-1743. doi: 10.1038/s12276-020-00511-9. Epub 2020 Oct 12. Exp Mol Med. 2020. PMID: 33046822 Free PMC article.
-
Osteopontin as a biomarker for osteosarcoma therapy and prognosis.Oncol Lett. 2019 Mar;17(3):2592-2598. doi: 10.3892/ol.2019.9905. Epub 2019 Jan 8. Oncol Lett. 2019. PMID: 30854034 Free PMC article. Review.
-
Radiosensitising Cancer Using Phosphatidylinositol-3-Kinase (PI3K), Protein Kinase B (AKT) or Mammalian Target of Rapamycin (mTOR) Inhibitors.Cancers (Basel). 2020 May 18;12(5):1278. doi: 10.3390/cancers12051278. Cancers (Basel). 2020. PMID: 32443649 Free PMC article. Review.
-
Alginate-based 3D cancer cell culture for therapeutic response modeling.STAR Protoc. 2021 Mar 18;2(2):100391. doi: 10.1016/j.xpro.2021.100391. eCollection 2021 Jun 18. STAR Protoc. 2021. PMID: 33778784 Free PMC article.
-
SPP1 Derived from Macrophages Is Associated with a Worse Clinical Course and Chemo-Resistance in Lung Adenocarcinoma.Cancers (Basel). 2022 Sep 8;14(18):4374. doi: 10.3390/cancers14184374. Cancers (Basel). 2022. PMID: 36139536 Free PMC article.
References
-
- Stephen AG, Esposito D, Bagni RK, McCormick F. Dragging ras back in the ring. Cancer Cell. 2014;25:272–81. - PubMed
-
- Eberhard DA, Johnson BE, Amler LC, Goddard AD, Heldens SL, Herbst RS, et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol. 2005;23:5900–9. - PubMed
-
- Han SW, Kim TY, Jeon YK, Hwang PG, Im SA, Lee KH, et al. Optimization of patient selection for gefitinib in non-small cell lung cancer by combined analysis of epidermal growth factor receptor mutation, K-ras mutation, and Akt phosphorylation. Clin Cancer Res. 2006;12:2538–44. - PubMed
-
- Tsao MS, Aviel-Ronen S, Ding K, Lau D, Liu N, Sakurada A, et al. Prognostic and predictive importance of p53 and RAS for adjuvant chemotherapy in non small-cell lung cancer. J Clin Oncol. 2007;25:5240–7. - PubMed
-
- Winton T, Livingston R, Johnson D, Rigas J, Johnston M, Butts C, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med. 2005;352:2589–97. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

