m6A-dependent maternal mRNA clearance facilitates zebrafish maternal-to-zygotic transition
- PMID: 28192787
- PMCID: PMC5323276
- DOI: 10.1038/nature21355
m6A-dependent maternal mRNA clearance facilitates zebrafish maternal-to-zygotic transition
Abstract
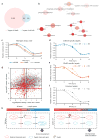
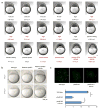
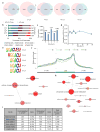
The maternal-to-zygotic transition (MZT) is one of the most profound and tightly orchestrated processes during the early life of embryos, yet factors that shape the temporal pattern of vertebrate MZT are largely unknown. Here we show that over one-third of zebrafish maternal messenger RNAs (mRNAs) can be N6-methyladenosine (m6A) modified, and the clearance of these maternal mRNAs is facilitated by an m6A-binding protein, Ythdf2. Removal of Ythdf2 in zebrafish embryos decelerates the decay of m6A-modified maternal mRNAs and impedes zygotic genome activation. These embryos fail to initiate timely MZT, undergo cell-cycle pause, and remain developmentally delayed throughout larval life. Our study reveals m6A-dependent RNA decay as a previously unidentified maternally driven mechanism that regulates maternal mRNA clearance during zebrafish MZT, highlighting the critical role of m6A mRNA methylation in transcriptome switching and animal development.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

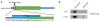



Comment in
-
RNA Methylation Clears the Way.Dev Cell. 2017 Mar 13;40(5):427-428. doi: 10.1016/j.devcel.2017.02.024. Dev Cell. 2017. PMID: 28292421
-
Mark it for destruction: a novel role of mRNA methylation in maternal-to-zygotic transition†.Biol Reprod. 2017 Apr 1;96(4):829-830. doi: 10.1093/biolre/iox016. Biol Reprod. 2017. PMID: 28371885 Free PMC article. No abstract available.
Similar articles
-
Ythdf m6A Readers Function Redundantly during Zebrafish Development.Cell Rep. 2020 Dec 29;33(13):108598. doi: 10.1016/j.celrep.2020.108598. Cell Rep. 2020. PMID: 33378672 Free PMC article.
-
RNA 5-Methylcytosine Facilitates the Maternal-to-Zygotic Transition by Preventing Maternal mRNA Decay.Mol Cell. 2019 Sep 19;75(6):1188-1202.e11. doi: 10.1016/j.molcel.2019.06.033. Epub 2019 Aug 6. Mol Cell. 2019. PMID: 31399345
-
Igf2bp3 maintains maternal RNA stability and ensures early embryo development in zebrafish.Commun Biol. 2020 Mar 3;3(1):94. doi: 10.1038/s42003-020-0827-2. Commun Biol. 2020. PMID: 32127635 Free PMC article.
-
RNA tales - how embryos read and discard messages from mom.J Cell Sci. 2018 Mar 1;131(5):jcs201996. doi: 10.1242/jcs.201996. J Cell Sci. 2018. PMID: 29467249 Review.
-
A story of birth and death: mRNA translation and clearance at the onset of maternal-to-zygotic transition in mammals†.Biol Reprod. 2019 Sep 1;101(3):579-590. doi: 10.1093/biolre/ioz012. Biol Reprod. 2019. PMID: 30715134 Review.
Cited by
-
Roles of N6-Methyladenosine (m6A) in Stem Cell Fate Decisions and Early Embryonic Development in Mammals.Front Cell Dev Biol. 2020 Aug 11;8:782. doi: 10.3389/fcell.2020.00782. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32850871 Free PMC article. Review.
-
From canonical to modified nucleotides: balancing translation and metabolism.Crit Rev Biochem Mol Biol. 2020 Dec;55(6):525-540. doi: 10.1080/10409238.2020.1818685. Epub 2020 Sep 16. Crit Rev Biochem Mol Biol. 2020. PMID: 32933330 Free PMC article. Review.
-
Regulation of mRNA Stability During Bacterial Stress Responses.Front Microbiol. 2020 Sep 9;11:2111. doi: 10.3389/fmicb.2020.02111. eCollection 2020. Front Microbiol. 2020. PMID: 33013770 Free PMC article. Review.
-
Deciphering Epitranscriptome: Modification of mRNA Bases Provides a New Perspective for Post-transcriptional Regulation of Gene Expression.Front Cell Dev Biol. 2021 Mar 16;9:628415. doi: 10.3389/fcell.2021.628415. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33816473 Free PMC article. Review.
-
Programmable m6A modification of cellular RNAs with a Cas13-directed methyltransferase.Nat Biotechnol. 2020 Dec;38(12):1431-1440. doi: 10.1038/s41587-020-0572-6. Epub 2020 Jun 29. Nat Biotechnol. 2020. PMID: 32601430 Free PMC article.
References
-
- Tadros W, Lipshitz HD. The maternal-to-zygotic transition: a play in two acts. Development. 2009;136:3033–3042. - PubMed
-
- Walser CB, Lipshitz HD. Transcript clearance during the maternal-to-zygotic transition. Curr Opin Genet Dev. 2011;21:431–443. - PubMed
-
- Giraldez AJ, et al. Zebrafish miR-430 promotes deadenylation and clearance of maternal mRNAs. Science. 2006;312:75–79. - PubMed
-
- Mishima Y, Tomari Y. Codon usage and 3′UTR length determine maternal mRNA stability in zebrafish. Mol Cell. 2016;61:874–885. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

