Development of a Three-Dimensional Bioengineering Technology to Generate Lung Tissue for Personalized Disease Modeling
- PMID: 28191779
- PMCID: PMC5442826
- DOI: 10.5966/sctm.2016-0192
Development of a Three-Dimensional Bioengineering Technology to Generate Lung Tissue for Personalized Disease Modeling
Abstract
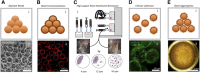
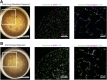
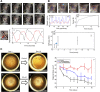
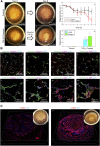
Stem cell technologies, especially patient-specific, induced stem cell pluripotency and directed differentiation, hold great promise for changing the landscape of medical therapies. Proper exploitation of these methods may lead to personalized organ transplants, but to regenerate organs, it is necessary to develop methods for assembling differentiated cells into functional, organ-level tissues. The generation of three-dimensional human tissue models also holds potential for medical advances in disease modeling, as full organ functionality may not be necessary to recapitulate disease pathophysiology. This is specifically true of lung diseases where animal models often do not recapitulate human disease. Here, we present a method for the generation of self-assembled human lung tissue and its potential for disease modeling and drug discovery for lung diseases characterized by progressive and irreversible scarring such as idiopathic pulmonary fibrosis (IPF). Tissue formation occurs because of the overlapping processes of cellular adhesion to multiple alveolar sac templates, bioreactor rotation, and cellular contraction. Addition of transforming growth factor-β1 to single cell-type mesenchymal organoids resulted in morphologic scarring typical of that seen in IPF but not in two-dimensional IPF fibroblast cultures. Furthermore, this lung organoid may be modified to contain multiple lung cell types assembled into the correct anatomical location, thereby allowing cell-cell contact and recapitulating the lung microenvironment. Our bottom-up approach for synthesizing patient-specific lung tissue in a scalable system allows for the development of relevant human lung disease models with the potential for high throughput drug screening to identify targeted therapies. Stem Cells Translational Medicine 2017;6:622-633.
Keywords: Disease modeling; Lung; Three-dimensional cell culture; Tissue engineering.
© 2016 The Authors Stem Cells Translational Medicine published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Figures
Similar articles
-
Disease modeling of pulmonary fibrosis using human pluripotent stem cell-derived alveolar organoids.Stem Cell Reports. 2021 Dec 14;16(12):2973-2987. doi: 10.1016/j.stemcr.2021.10.015. Epub 2021 Nov 18. Stem Cell Reports. 2021. PMID: 34798066 Free PMC article.
-
Disease Modeling Using 3D Organoids Derived from Human Induced Pluripotent Stem Cells.Int J Mol Sci. 2018 Mar 21;19(4):936. doi: 10.3390/ijms19040936. Int J Mol Sci. 2018. PMID: 29561796 Free PMC article. Review.
-
Human iPSC-Based Modeling of Central Nerve System Disorders for Drug Discovery.Int J Mol Sci. 2021 Jan 26;22(3):1203. doi: 10.3390/ijms22031203. Int J Mol Sci. 2021. PMID: 33530458 Free PMC article. Review.
-
Transcription factor GATA-6 is expressed in quiescent myofibroblasts in idiopathic pulmonary fibrosis.Am J Respir Cell Mol Biol. 2010 May;42(5):626-32. doi: 10.1165/rcmb.2009-0021OC. Epub 2009 Jul 13. Am J Respir Cell Mol Biol. 2010. PMID: 19597127
-
Human airway organoid engineering as a step toward lung regeneration and disease modeling.Biomaterials. 2017 Jan;113:118-132. doi: 10.1016/j.biomaterials.2016.10.046. Epub 2016 Oct 28. Biomaterials. 2017. PMID: 27815996 Free PMC article.
Cited by
-
Through the Looking Glass: In Vitro Models for Inhalation Toxicology and Interindividual Variability in the Airway.Appl In Vitro Toxicol. 2018 Jun 1;4(2):115-128. doi: 10.1089/aivt.2018.0002. Appl In Vitro Toxicol. 2018. PMID: 31380467 Free PMC article. Review.
-
Bioreactor Technologies for Enhanced Organoid Culture.Int J Mol Sci. 2023 Jul 13;24(14):11427. doi: 10.3390/ijms241411427. Int J Mol Sci. 2023. PMID: 37511186 Free PMC article. Review.
-
Alveolar Organoids in Lung Disease Modeling.Biomolecules. 2024 Jan 16;14(1):115. doi: 10.3390/biom14010115. Biomolecules. 2024. PMID: 38254715 Free PMC article. Review.
-
Induced Pluripotent Stem Cell-Derived Organoids: Their Implication in COVID-19 Modeling.Int J Mol Sci. 2023 Feb 9;24(4):3459. doi: 10.3390/ijms24043459. Int J Mol Sci. 2023. PMID: 36834870 Free PMC article. Review.
-
Organoids as preclinical models of human disease: progress and applications.Med Rev (2021). 2024 Mar 14;4(2):129-153. doi: 10.1515/mr-2023-0047. eCollection 2024 Apr. Med Rev (2021). 2024. PMID: 38680680 Free PMC article. Review.
References
-
- NIH. NHLBI. Chapter 4 Disease Statistics. NHLBI FACT BOOK, FISC. YEAR 2012 2012:33–52. Available at: http://www.nhlbi.nih.gov/about/factbook/chapter4.htm#gr36\nhttp://www.nhlbi.nih.gov/about/documents/factbook/2012. Accessed November 10, 2015
-
- Selman M, King TE, Pardo A; American Thoracic Society; European Respiratory Society; American College of Chest Physicians. Idiopathic pulmonary fibrosis: Prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med 2001;134:136–151. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

