Xanthohumol isolated from Humulus lupulus prevents thrombosis without increased bleeding risk by inhibiting platelet activation and mtDNA release
- PMID: 28188927
- PMCID: PMC5508526
- DOI: 10.1016/j.freeradbiomed.2017.02.018
Xanthohumol isolated from Humulus lupulus prevents thrombosis without increased bleeding risk by inhibiting platelet activation and mtDNA release
Abstract
Aim: As the global population has reached 7 billion and the baby boom generation reaches old age, thrombosis has become the major contributor to the global disease burden. It has been reported that, in moderate doses, beer may protect against thrombosis. Xanthohumol (XN), an antioxidant, is found at high concentrations in hop cones (Humulus lupulus L.) and is a common ingredient of beer. Here, the aim of the present work was to investigate the effects of XN on antithrombotic and antiplatelet activities, and study its mechanism.
Approach and results: Using ferric chloride-induced carotid artery injury, inferior vena cava ligation model, and platelet function tests, we demonstrated that XN uniquely prevents both venous and arterial thrombosis by inhibiting platelet activation. Interestingly, in tail bleeding time studies, XN did not increase bleeding risk, which is recognized as a major limitation of current antithrombotic therapies. We also demonstrated that XN induces Sirt1 expression and thereby decreases reactive oxygen species (ROS) overload, prevents mitochondrial dysfunction, and reduces activated platelet-induced mitochondrial hyperpolarization, respiratory disorders, and associated membrane damage at low concentrations. In mitochondrial function assays designed to detect amounts of extracellular mitochondrial DNA (mtDNA), we found that XN prevents mtDNA release, which induces platelet activation in a DC-SIGN-dependent manner.
Conclusions: XN exemplifies a promising new class of antiplatelet agents that are highly effective at inhibiting platelet activation by decreasing ROS accumulation and platelet mtDNA release without incurring a bleeding risk. This study has also provided novel insights into mechanisms of thrombotic diseases with possible therapeutic implications.
Keywords: Beer; Bleeding risk; Mitochondrial DNA; Thrombosis; Xanthohumol.
Copyright © 2017. Published by Elsevier Inc.
Conflict of interest statement
No conflicts of interest to declare.
Figures
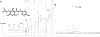
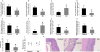
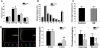

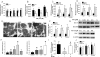
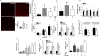
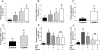
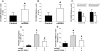
Similar articles
-
Xanthohumol from hop cones (Humulus lupulus L.) prevents ADP-induced platelet reactivity.Arch Physiol Biochem. 2017 Feb;123(1):54-60. doi: 10.1080/13813455.2016.1247284. Epub 2016 Nov 18. Arch Physiol Biochem. 2017. PMID: 27855519
-
Danshensu prevents thrombosis by inhibiting platelet activation via SIRT1/ROS/mtDNA pathways without increasing bleeding risk.Phytomedicine. 2022 Sep;104:154271. doi: 10.1016/j.phymed.2022.154271. Epub 2022 Jun 12. Phytomedicine. 2022. PMID: 35777120
-
Metformin Uniquely Prevents Thrombosis by Inhibiting Platelet Activation and mtDNA Release.Sci Rep. 2016 Nov 2;6:36222. doi: 10.1038/srep36222. Sci Rep. 2016. PMID: 27805009 Free PMC article.
-
Pharmacological profile of xanthohumol, a prenylated flavonoid from hops (Humulus lupulus).Molecules. 2015 Jan 7;20(1):754-79. doi: 10.3390/molecules20010754. Molecules. 2015. PMID: 25574819 Free PMC article. Review.
-
Recent patents on therapeutic activities of xanthohumol: a prenylated chalconoid from hops (Humulus lupulus L.).Pharm Pat Anal. 2021 Jan;10(1):37-49. doi: 10.4155/ppa-2020-0026. Epub 2021 Jan 15. Pharm Pat Anal. 2021. PMID: 33445965 Review.
Cited by
-
Xanthohumol-A Miracle Molecule with Biological Activities: A Review of Biodegradable Polymeric Carriers and Naturally Derived Compounds for Its Delivery.Int J Mol Sci. 2024 Mar 17;25(6):3398. doi: 10.3390/ijms25063398. Int J Mol Sci. 2024. PMID: 38542371 Free PMC article. Review.
-
Integrating Phenotypic and Chemoproteomic Approaches to Identify Covalent Targets of Dietary Electrophiles in Platelets.ACS Cent Sci. 2024 Jan 29;10(2):344-357. doi: 10.1021/acscentsci.3c00822. eCollection 2024 Feb 28. ACS Cent Sci. 2024. PMID: 38435523 Free PMC article.
-
The Antimicrobial Potential of the Hop (Humulus lupulus L.) Extract against Staphylococcus aureus and Oral Streptococci.Pharmaceuticals (Basel). 2024 Jan 27;17(2):162. doi: 10.3390/ph17020162. Pharmaceuticals (Basel). 2024. PMID: 38399377 Free PMC article.
-
Phloretin targets SIRT1 to alleviate oxidative stress, apoptosis, and inflammation in deep venous thrombosis.Toxicol Res. 2023 Aug 30;40(1):83-96. doi: 10.1007/s43188-023-00207-y. eCollection 2024 Jan. Toxicol Res. 2023. PMID: 38223667
-
Humulus lupus extract rich in xanthohumol improves the clinical course in critically ill COVID-19 patients.Biomed Pharmacother. 2023 Feb;158:114082. doi: 10.1016/j.biopha.2022.114082. Epub 2022 Dec 9. Biomed Pharmacother. 2023. PMID: 36508996 Free PMC article. Clinical Trial.
References
-
- Creager MA, Lüscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease pathophysiology, clinical consequences, and medical therapy: part I. Circulation. 2003;108:1527–1532. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

