Rediscovering the octapeptins
- PMID: 28180225
- PMCID: PMC5802877
- DOI: 10.1039/c6np00113k
Rediscovering the octapeptins
Abstract
Covering: 1975 up to the end of 2016The decline in the discovery and development of novel antibiotics has resulted in the emergence of bacteria that are resistant to almost all available antibiotics. Currently, polymyxin B and E (colistin) are being used as the last-line therapy against life-threatening infections, unfortunately resistance to polymyxins in both the community and hospital setting is becoming more common. Octapeptins are structurally related non-ribosomal lipopeptide antibiotics that do not exhibit cross-resistance with polymyxins and have a broader spectrum of activity that includes Gram-positive bacteria. This makes them a precious and finite resource for the development of new antibiotics against these problematic polymyxin-resistant Gram-negative pathogens, in particular Pseudomonas aeruginosa, Acinetobacter baumannii and Klebsiella pneumoniae. This review surveys the progress in understanding octapeptin chemistry, mechanisms of antibacterial activity and biosynthesis. With the lack of cross-resistance and their broad antibacterial activity, the octapeptins represent ideal candidates for the development of a new generation of polymyxin-like lipopeptide antibiotics targeting polymyxin-resistant 'superbugs'.
Figures
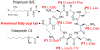

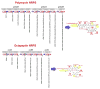
Similar articles
-
Octapeptin C4 and polymyxin resistance occur via distinct pathways in an epidemic XDR Klebsiella pneumoniae ST258 isolate.J Antimicrob Chemother. 2019 Mar 1;74(3):582-593. doi: 10.1093/jac/dky458. J Antimicrob Chemother. 2019. PMID: 30445429 Free PMC article.
-
History, Chemistry and Antibacterial Spectrum.Adv Exp Med Biol. 2019;1145:15-36. doi: 10.1007/978-3-030-16373-0_3. Adv Exp Med Biol. 2019. PMID: 31364069 Review.
-
A Whole-Cell Screen Identifies Small Bioactives That Synergize with Polymyxin and Exhibit Antimicrobial Activities against Multidrug-Resistant Bacteria.Antimicrob Agents Chemother. 2020 Feb 21;64(3):e01677-19. doi: 10.1128/AAC.01677-19. Print 2020 Feb 21. Antimicrob Agents Chemother. 2020. PMID: 31844003 Free PMC article.
-
Synergistic combinations of polymyxins.Int J Antimicrob Agents. 2016 Dec;48(6):607-613. doi: 10.1016/j.ijantimicag.2016.09.014. Epub 2016 Oct 24. Int J Antimicrob Agents. 2016. PMID: 27865626 Free PMC article. Review.
-
Teaching 'old' polymyxins new tricks: new-generation lipopeptides targeting gram-negative 'superbugs'.ACS Chem Biol. 2014 May 16;9(5):1172-7. doi: 10.1021/cb500080r. Epub 2014 Mar 17. ACS Chem Biol. 2014. PMID: 24601489 Free PMC article.
Cited by
-
Octapeptin C4 and polymyxin resistance occur via distinct pathways in an epidemic XDR Klebsiella pneumoniae ST258 isolate.J Antimicrob Chemother. 2019 Mar 1;74(3):582-593. doi: 10.1093/jac/dky458. J Antimicrob Chemother. 2019. PMID: 30445429 Free PMC article.
-
Simulations of octapeptin-outer membrane interactions reveal conformational flexibility is linked to antimicrobial potency.J Biol Chem. 2020 Nov 20;295(47):15902-15912. doi: 10.1074/jbc.RA120.014856. Epub 2020 Sep 10. J Biol Chem. 2020. PMID: 32913118 Free PMC article.
-
Strategic Moves of "Superbugs" Against Available Chemical Scaffolds: Signaling, Regulation, and Challenges.ACS Pharmacol Transl Sci. 2020 Apr 13;3(3):373-400. doi: 10.1021/acsptsci.0c00005. eCollection 2020 Jun 12. ACS Pharmacol Transl Sci. 2020. PMID: 32566906 Free PMC article. Review.
-
Can octapeptin antibiotics combat extensively drug-resistant (XDR) bacteria?Expert Rev Anti Infect Ther. 2018 Jun;16(6):485-499. doi: 10.1080/14787210.2018.1483240. Epub 2018 Jun 26. Expert Rev Anti Infect Ther. 2018. PMID: 29848132 Free PMC article. Review.
-
Recent Advances in the Development of Polymyxin Antibiotics: 2010-2023.ACS Infect Dis. 2024 Apr 12;10(4):1056-1079. doi: 10.1021/acsinfecdis.3c00630. Epub 2024 Mar 12. ACS Infect Dis. 2024. PMID: 38470446 Free PMC article. Review.
References
-
- C. f. D. C. a. Prevention. Antibiotic resistance threats in the United State. 2013.
-
- Shlaes DM. Antibiotics. 2010. pp. 97–99. - DOI
-
- Shlaes DM, Spellberg B. Curr Opin Pharmacol. 2012;12:522–526. - PubMed
-
- IDSA. [last accessed on April 29, 2008];Bad bugs, no drugs. http://www.idsociety.org/badbugsnodrugs.html.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

