Prime-boost immunization by both DNA vaccine and oncolytic adenovirus expressing GM-CSF and shRNA of TGF-β2 induces anti-tumor immune activation
- PMID: 28178658
- PMCID: PMC5362529
- DOI: 10.18632/oncotarget.15008
Prime-boost immunization by both DNA vaccine and oncolytic adenovirus expressing GM-CSF and shRNA of TGF-β2 induces anti-tumor immune activation
Abstract
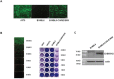
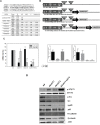
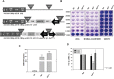
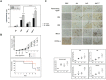
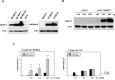
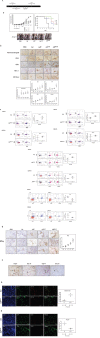
A successful DNA vaccine for the treatment of tumors should break established immune tolerance to tumor antigen. However, due to the relatively low immunogenicity of DNA vaccines, compared to other kinds of vaccines using live virus or protein, a recombinant viral vector was used to enhance humoral and cellular immunity. In the current study, we sought to develop a novel anti-cancer agent as a complex of DNA and oncolytic adenovirus for the treatment of malignant melanoma in the C57BL/6 mouse model. MART1, a human melanoma-specific tumor antigen, was used to induce an increased immune reaction, since a MART1-protective response is required to overcome immune tolerance to the melanoma antigen MelanA. Because GM-CSF is a potent inducer of anti-tumor immunity and TGF-β2 is involved in tumor survival and host immune suppression, mouse GM-CSF (mGM-CSF) and shRNA of mouse TGF-β2 (shmTGF-β2) genes were delivered together with MART1 via oncolytic adenovirus. MART1 plasmid was also used for antigen-priming. To compare the anti-tumor effect of oncolytic adenovirus expressing both mGM-CSF and shmTGF-β2 (AdGshT) with that of oncolytic adenovirus expressing mGM-CSF only (AdG), each virus was intratumorally injected into melanoma-bearing C57BL/6 mice. As a result, mice that received AdGshT showed delayed tumor growth than those that received AdG. Heterologous prime-boost immunization was combined with oncolytic AdGshT and MART1 expression to result in further delayed tumor growth. This regression is likely due to the following 4 combinations: MART1-derived mouse melanoma antigen-specific immune reaction, immune stimulation by mGM-CSF/shmTGF-β2, tumor growth inhibition by shmTGF-β2, and tumor cell-specific lysis via an oncolytic adenovirus. Immune activation was mainly induced by mature tumor-infiltrating dendritic cell (TIDC) and lowered regulatory T cells in tumor-infiltrating lymphocytes (TIL). Taken together, these findings demonstrate that human MART1 induces a mouse melanoma antigen-specific immune reaction. In addition, the results also indicate that combination therapy of MART1 plasmid, together with an oncolytic adenovirus expressing MART1, mGM-CSF, and shmTGF-β2, is a promising candidate for the treatment of malignant melanoma.
Keywords: DNA vaccine; GM-CSF; MART1; TGF-β2; oncolytic adenovirus.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Concurrent delivery of GM-CSF and B7-1 using an oncolytic adenovirus elicits potent antitumor effect.Gene Ther. 2006 Jul;13(13):1010-20. doi: 10.1038/sj.gt.3302759. Epub 2006 Mar 9. Gene Ther. 2006. PMID: 16525479
-
An Oncolytic Adenovirus Encoding Decorin and Granulocyte Macrophage Colony Stimulating Factor Inhibits Tumor Growth in a Colorectal Tumor Model by Targeting Pro-Tumorigenic Signals and via Immune Activation.Hum Gene Ther. 2017 Aug;28(8):667-680. doi: 10.1089/hum.2017.033. Hum Gene Ther. 2017. PMID: 28530155
-
Antigen-specific tumor vaccines. Development and characterization of recombinant adenoviruses encoding MART1 or gp100 for cancer therapy.J Immunol. 1996 Jan 15;156(2):700-10. J Immunol. 1996. PMID: 8543823
-
Current status of granulocyte-macrophage colony-stimulating factor in the immunotherapy of melanoma.J Immunother Cancer. 2014 May 13;2:11. doi: 10.1186/2051-1426-2-11. eCollection 2014. J Immunother Cancer. 2014. PMID: 24971166 Free PMC article. Review.
-
Cancer-Targeted Oncolytic Adenoviruses for Modulation of the Immune System.Curr Cancer Drug Targets. 2018;18(2):124-138. doi: 10.2174/1568009617666170502152352. Curr Cancer Drug Targets. 2018. PMID: 28464762 Review.
Cited by
-
Recombinant Viral Vectors for Therapeutic Programming of Tumour Microenvironment: Advantages and Limitations.Biomedicines. 2022 Aug 31;10(9):2142. doi: 10.3390/biomedicines10092142. Biomedicines. 2022. PMID: 36140243 Free PMC article. Review.
-
Combination Immunotherapy with Vaccine and Oncolytic HSV Virotherapy Is Time Dependent.Mol Cancer Ther. 2024 Sep 4;23(9):1273-1281. doi: 10.1158/1535-7163.MCT-23-0873. Mol Cancer Ther. 2024. PMID: 38710101 Free PMC article.
-
In situ administration of cytokine combinations induces tumor regression in mice.EBioMedicine. 2018 Nov;37:38-46. doi: 10.1016/j.ebiom.2018.09.050. Epub 2018 Oct 5. EBioMedicine. 2018. PMID: 30297145 Free PMC article.
-
Incorporation of a TGF-β2-inhibiting oligodeoxynucleotide molecular adjuvant into a tumor cell lysate vaccine to enhance antiglioma immunity in mice.Front Immunol. 2023 Jan 27;14:1013342. doi: 10.3389/fimmu.2023.1013342. eCollection 2023. Front Immunol. 2023. PMID: 36776837 Free PMC article.
-
Synergistic antitumor effect of the combination of a dual cancer-specific oncolytic adenovirus and cisplatin on lung cancer cells.Oncol Lett. 2018 Nov;16(5):6275-6282. doi: 10.3892/ol.2018.9470. Epub 2018 Sep 20. Oncol Lett. 2018. PMID: 30405762 Free PMC article.
References
-
- Rigel DS, Carucci JA. Malignant melanoma: prevention, early detection, and treatment in the 21st century. CA Cancer J Clin. 2000;50:215–236. quiz 237-240. - PubMed
-
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. - PubMed
-
- Jordan EJ, Kelly CM. Vemurafenib for the treatment of melanoma. Expert opinion on pharmacotherapy. 2012;13:2533–2543. - PubMed
-
- Trinh VA, Hwu WJ. Ipilimumab in the treatment of melanoma. Expert opinion on biological therapy. 2012;12:773–782. - PubMed
-
- Starz H. Ipilimumab for advanced metastatic melanoma. Expert opinion on biological therapy. 2012;12:981–982. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

