A randomized placebo-controlled phase II study of a Pseudomonas vaccine in ventilated ICU patients
- PMID: 28159015
- PMCID: PMC5291979
- DOI: 10.1186/s13054-017-1601-9
A randomized placebo-controlled phase II study of a Pseudomonas vaccine in ventilated ICU patients
Abstract
Background: Currently, no vaccine against Pseudomonas is available. IC43 is a new, recombinant, protein (OprF/I)-based vaccine against the opportunistic pathogen, Pseudomonas aeruginosa, a major cause of serious hospital-acquired infections. IC43 has proven immunogenicity and tolerability in healthy volunteers, patients with burns, and patients with chronic lung diseases. In order to assess the immunogenicity and safety of IC43 in patients who are most at risk of acquiring Pseudomonas infections, it was evaluated in mechanically ventilated ICU patients.
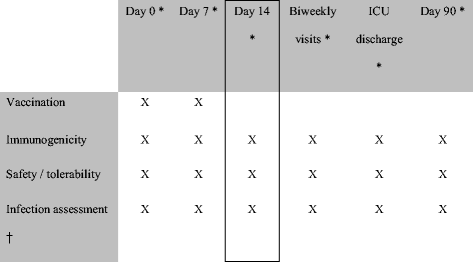
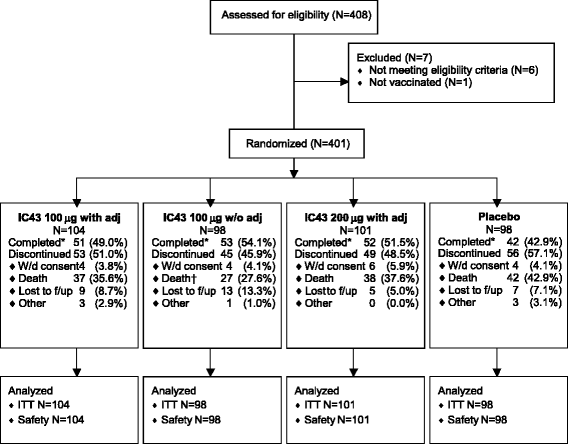
Methods: We conducted a randomized, placebo-controlled, partially blinded study in mechanically ventilated ICU patients. The immunogenicity of IC43 at day 14 was determined as the primary endpoint, and safety, efficacy against P. aeruginosa infections, and all-cause mortality were evaluated as secondary endpoints. Vaccinations (100 μg or 200 μg IC43 with adjuvant, or 100 μg IC43 without adjuvant, or placebo) were given twice in a 7-day interval and patients were followed up for 90 days.
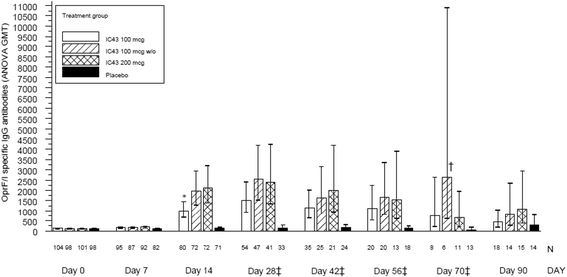
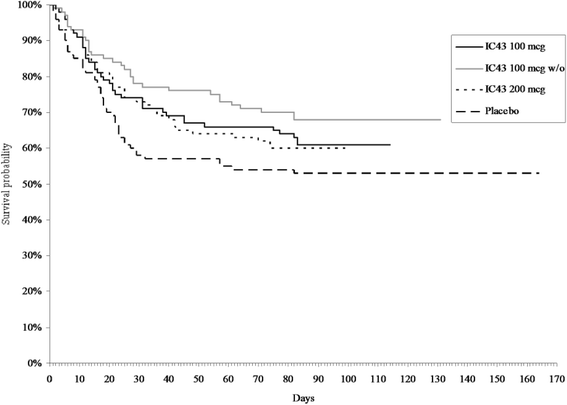
Results: Higher OprF/I IgG antibody titers were seen at day 14 for all IC43 groups versus placebo (P < 0.0001). Seroconversion (≥4-fold increase in OprF/I IgG titer from days 0 to 14) was highest with 100 μg IC43 without adjuvant (80.6%). There were no significant differences in P. aeruginosa infection rates, with a low rate of invasive infections (pneumonia or bacteremia) in the IC43 groups (11.2-14.0%). Serious adverse events (SAEs) considered possibly related to therapy were reported by 2 patients (1.9%) in the group of 100 µg IC43 with adjuvant. Both SAEs resolved and no deaths were related to study treatment. Local tolerability symptoms were mild and rare (<5% of patients), a low rate of treatment-related treatment-emergent adverse events (3.1-10.6%) was observed in the IC43 groups.
Conclusion: This phase II study has shown that IC43 vaccination of ventilated ICU patients produced a significant immunogenic effect. P. aeruginosa infection rates did not differ significantly between groups. In the absence of any difference in immune response following administration of 100 μg IC43 without adjuvant compared with 200 μg IC43 with adjuvant, the 100 μg dose without adjuvant was considered for further testing of its possible benefit of improved outcomes. There were no safety or mortality concerns.
Trial registration: ClinicalTrials.gov, NCT00876252 . Registered on 3 April 2009.
Keywords: Bacterial infections; Immunity; Immunocompromised host; Mortality; Pseudomonas aeruginosa; Vaccination.
Figures
Comment in
-
A brief comment on vaccinations for opportunistic microorganisms.Crit Care. 2017 May 5;21(1):100. doi: 10.1186/s13054-017-1684-3. Crit Care. 2017. PMID: 28472994 Free PMC article. No abstract available.
Similar articles
-
Efficacy, immunogenicity, and safety of IC43 recombinant Pseudomonas aeruginosa vaccine in mechanically ventilated intensive care patients-a randomized clinical trial.Crit Care. 2020 Mar 4;24(1):74. doi: 10.1186/s13054-020-2792-z. Crit Care. 2020. PMID: 32131866 Free PMC article. Clinical Trial.
-
A randomized, placebo-controlled phase I study assessing the safety and immunogenicity of a Pseudomonas aeruginosa hybrid outer membrane protein OprF/I vaccine (IC43) in healthy volunteers.Hum Vaccin Immunother. 2014;10(1):170-83. doi: 10.4161/hv.26565. Epub 2013 Sep 24. Hum Vaccin Immunother. 2014. PMID: 24064511 Free PMC article. Clinical Trial.
-
Safety, efficacy, and pharmacokinetics of gremubamab (MEDI3902), an anti-Pseudomonas aeruginosa bispecific human monoclonal antibody, in P. aeruginosa-colonised, mechanically ventilated intensive care unit patients: a randomised controlled trial.Crit Care. 2022 Nov 15;26(1):355. doi: 10.1186/s13054-022-04204-9. Crit Care. 2022. PMID: 36380312 Free PMC article. Clinical Trial.
-
Immunological considerations in the development of Pseudomonas aeruginosa vaccines.Hum Vaccin Immunother. 2020;16(2):412-418. doi: 10.1080/21645515.2019.1650999. Epub 2019 Sep 5. Hum Vaccin Immunother. 2020. PMID: 31368828 Free PMC article. Review.
-
Pseudomonas aeruginosa: the potential to immunise against infection.Expert Opin Biol Ther. 2005 Jul;5(7):967-82. doi: 10.1517/14712598.5.7.967. Expert Opin Biol Ther. 2005. PMID: 16018741 Review.
Cited by
-
What Is New in the Anti-Pseudomonas aeruginosa Clinical Development Pipeline Since the 2017 WHO Alert?Front Cell Infect Microbiol. 2022 Jul 8;12:909731. doi: 10.3389/fcimb.2022.909731. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35880080 Free PMC article. Review.
-
Advances in Development of Novel Therapeutic Strategies against Multi-Drug Resistant Pseudomonas aeruginosa.Antibiotics (Basel). 2024 Jan 25;13(2):119. doi: 10.3390/antibiotics13020119. Antibiotics (Basel). 2024. PMID: 38391505 Free PMC article. Review.
-
Epidemiology and Treatment of Multidrug-Resistant and Extensively Drug-Resistant Pseudomonas aeruginosa Infections.Clin Microbiol Rev. 2019 Aug 28;32(4):e00031-19. doi: 10.1128/CMR.00031-19. Print 2019 Sep 18. Clin Microbiol Rev. 2019. PMID: 31462403 Free PMC article. Review.
-
Construction of a Protective Vaccine Against Lipopolysaccharide-Heterologous Pseudomonas aeruginosa Strains Based on Expression Profiling of Outer Membrane Proteins During Infection.Front Immunol. 2018 Jul 26;9:1737. doi: 10.3389/fimmu.2018.01737. eCollection 2018. Front Immunol. 2018. PMID: 30093906 Free PMC article.
-
Progress towards the development of Klebsiella vaccines.Expert Rev Vaccines. 2019 Jul;18(7):681-691. doi: 10.1080/14760584.2019.1635460. Epub 2019 Jun 28. Expert Rev Vaccines. 2019. PMID: 31250679 Free PMC article. Review.
References
-
- Rosenthal VD, Maki DG, Jamulitrat S, Medeiros EA, Todi SK, Gomez DY, Leblebicioglu H, Khader IA, Novales MGM, Berba R, Wong FMR, Barkat A, Pino OR, Dueñas L, Mitrev Z, Bijie H, Gurskis V, Kanj SS, Mapp T, Hidalgo RF, Jaballah NB, Raka L, Gikas A, Ahmed A, Thu LTA, Siritt MEG, INICC members International Nosocomial Infection Control Consortium (INICC) report, data summary for 2003–2008, issued June 2009. Am J Infect Control. 2010;38:95–106. doi: 10.1016/j.ajic.2009.12.004. - DOI - PubMed
-
- Apostolopoulou E, Bakakos P, Katostaras T, Gregorakos L. Incidence and risk factors for ventilator-associated pneumonia in 4 multidisciplinary intensive care units in Athens. Greece Respir Care. 2003;48(7):681–8. - PubMed
-
- Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, Moreno R, Lipman J, Gomersall C, Sakr Y, Reinhart K, EPIC II group of investigators International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302(21):2323. doi: 10.1001/jama.2009.1754. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

