Gene polymorphism linked to increased asthma and IBD risk alters gasdermin-B structure, a sulfatide and phosphoinositide binding protein
- PMID: 28154144
- PMCID: PMC5321033
- DOI: 10.1073/pnas.1616783114
Gene polymorphism linked to increased asthma and IBD risk alters gasdermin-B structure, a sulfatide and phosphoinositide binding protein
Abstract
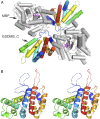
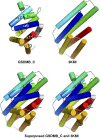
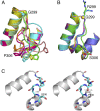
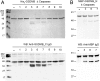
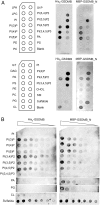
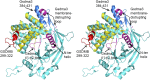
The exact function of human gasdermin-B (GSDMB), which regulates differentiation and growth of epithelial cells, is yet to be elucidated. In human epidermal growth factor receptor 2 (HER2)-positive breast cancer, GSDMB gene amplification and protein overexpression indicate a poor response to HER2-targeted therapy. Genome-wide association studies revealed a correlation between GSDMB SNPs and an increased susceptibility to Crohn's disease, ulcerative colitis, and asthma. The N- and C-terminal domains of all gasdermins possess lipid-binding and regulatory activities, respectively. Inflammatory caspases cleave gasdermin-D in the interdomain linker but not GSDMB. The cleaved N-terminal domain binds phosphoinositides and cardiolipin, forms membrane-disrupting pores, and executes pyroptosis. We show that both full-length GSDMB and the N-terminal domain bind to nitrocellulose membranes immobilized with phosphoinositides or sulfatide, but not with cardiolipin. In addition, the GSDMB N-terminal domain binds liposomes containing sulfatide. The crystal structure of the GSDMB C-terminal domain reveals the structural impact of the amino acids encoded by SNPs that are linked to asthma and inflammatory bowel disease (IBD). A loop that carries the polymorphism amino acids corresponding to healthy individuals (Gly299:Pro306) exhibits high conformational flexibility, whereas the loop carrying amino acids found in individuals with increased disease risk (Arg299:Ser306) exhibits a well-defined conformation and higher positive surface charge. Apoptotic executioner caspase-3, -6, and -7, but not the inflammatory caspases, cleave GSDMB at 88DNVD91 within the N-terminal domain. Selective sulfatide binding may indicate possible function for GSDMB in the cellular sulfatide transport.
Keywords: GSDMB; X-ray crystallography; complex trait inflammatory disease; disease risk polymorphism; lipid binding.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Comment in
-
Autoimmune disease variants regulate GSDMB gene expression in human immune cells and whole blood.Proc Natl Acad Sci U S A. 2017 Sep 19;114(38):E7860-E7862. doi: 10.1073/pnas.1712127114. Epub 2017 Sep 7. Proc Natl Acad Sci U S A. 2017. PMID: 28882878 Free PMC article. No abstract available.
-
Reply to HU et al.: On the interpretation of gasdermin-B expression quantitative trait loci data.Proc Natl Acad Sci U S A. 2017 Sep 19;114(38):E7863-E7864. doi: 10.1073/pnas.1712734114. Epub 2017 Sep 7. Proc Natl Acad Sci U S A. 2017. PMID: 28882987 Free PMC article. No abstract available.
Similar articles
-
Distinct GSDMB protein isoforms and protease cleavage processes differentially control pyroptotic cell death and mitochondrial damage in cancer cells.Cell Death Differ. 2023 May;30(5):1366-1381. doi: 10.1038/s41418-023-01143-y. Epub 2023 Mar 11. Cell Death Differ. 2023. PMID: 36899106 Free PMC article.
-
Pore-forming activity and structural autoinhibition of the gasdermin family.Nature. 2016 Jul 7;535(7610):111-6. doi: 10.1038/nature18590. Epub 2016 Jun 8. Nature. 2016. PMID: 27281216
-
A functional splice variant associated with decreased asthma risk abolishes the ability of gasdermin B to induce epithelial cell pyroptosis.J Allergy Clin Immunol. 2018 Nov;142(5):1469-1478.e2. doi: 10.1016/j.jaci.2017.11.040. Epub 2018 Jan 9. J Allergy Clin Immunol. 2018. PMID: 29330013 Free PMC article.
-
New Insights Relating Gasdermin B to the Onset of Childhood Asthma.Am J Respir Cell Mol Biol. 2022 Oct;67(4):430-437. doi: 10.1165/rcmb.2022-0043PS. Am J Respir Cell Mol Biol. 2022. PMID: 35580164 Free PMC article. Review.
-
Chromosome 17q21 Genes ORMDL3 and GSDMB in Asthma and Immune Diseases.Adv Immunol. 2017;135:1-52. doi: 10.1016/bs.ai.2017.06.001. Epub 2017 Jul 19. Adv Immunol. 2017. PMID: 28826527 Review.
Cited by
-
Advancing Roles and Therapeutic Potentials of Pyroptosis in Host Immune Defenses against Tuberculosis.Biomolecules. 2024 Oct 4;14(10):1255. doi: 10.3390/biom14101255. Biomolecules. 2024. PMID: 39456188 Free PMC article. Review.
-
Pyroptosis in Liver Disease: New Insights into Disease Mechanisms.Aging Dis. 2019 Oct 1;10(5):1094-1108. doi: 10.14336/AD.2019.0116. eCollection 2019 Oct. Aging Dis. 2019. PMID: 31595205 Free PMC article. Review.
-
Distinct GSDMB protein isoforms and protease cleavage processes differentially control pyroptotic cell death and mitochondrial damage in cancer cells.Cell Death Differ. 2023 May;30(5):1366-1381. doi: 10.1038/s41418-023-01143-y. Epub 2023 Mar 11. Cell Death Differ. 2023. PMID: 36899106 Free PMC article.
-
Role of gasdermin family proteins in cancers (Review).Int J Oncol. 2023 Sep;63(3):100. doi: 10.3892/ijo.2023.5548. Epub 2023 Jul 21. Int J Oncol. 2023. PMID: 37477150 Free PMC article.
-
Gasdermins assemble; recent developments in bacteriology and pharmacology.Front Immunol. 2023 May 17;14:1173519. doi: 10.3389/fimmu.2023.1173519. eCollection 2023. Front Immunol. 2023. PMID: 37266429 Free PMC article. Review.
References
-
- Tamura M, et al. Members of a novel gene family, Gsdm, are expressed exclusively in the epithelium of the skin and gastrointestinal tract in a highly tissue-specific manner. Genomics. 2007;89(5):618–629. - PubMed
-
- Carl-McGrath S, Schneider-Stock R, Ebert M, Röcken C. Differential expression and localisation of gasdermin-like (GSDML), a novel member of the cancer-associated GSDMDC protein family, in neoplastic and non-neoplastic gastric, hepatic, and colon tissues. Pathology. 2008;40(1):13–24. - PubMed
-
- Komiyama H, et al. Alu-derived cis-element regulates tumorigenesis-dependent gastric expression of GASDERMIN B (GSDMB) Genes Genet Syst. 2010;85(1):75–83. - PubMed
-
- Saeki N, et al. GASDERMIN, suppressed frequently in gastric cancer, is a target of LMO1 in TGF-beta-dependent apoptotic signalling. Oncogene. 2007;26(45):6488–6498. - PubMed
-
- Saeki N, Kuwahara Y, Sasaki H, Satoh H, Shiroishi T. Gasdermin (Gsdm) localizing to mouse Chromosome 11 is predominantly expressed in upper gastrointestinal tract but significantly suppressed in human gastric cancer cells. Mamm Genome. 2000;11(9):718–724. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

