Oral Tolerance Induction in Hemophilia B Dogs Fed with Transplastomic Lettuce
- PMID: 28153098
- PMCID: PMC5368425
- DOI: 10.1016/j.ymthe.2016.11.009
Oral Tolerance Induction in Hemophilia B Dogs Fed with Transplastomic Lettuce
Abstract
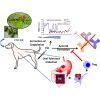
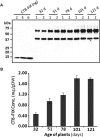
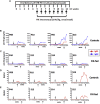
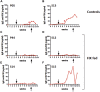
Anti-drug antibodies in hemophilia patients substantially complicate treatment. Their elimination through immune tolerance induction (ITI) protocols poses enormous costs, and ITI is often ineffective for factor IX (FIX) inhibitors. Moreover, there is no prophylactic ITI protocol to prevent anti-drug antibody (ADA) formation. Using general immune suppression is problematic. To address this urgent unmet medical need, we delivered antigen bioencapsulated in plant cells to hemophilia B dogs. Commercial-scale production of CTB-FIX fusion expressed in lettuce chloroplasts was done in a hydroponic facility. CTB-FIX (∼1 mg/g) in lyophilized cells was stable with proper folding, disulfide bonds, and pentamer assembly after 30-month storage at ambient temperature. Robust suppression of immunoglobulin G (IgG)/inhibitor and IgE formation against intravenous FIX was observed in three of four hemophilia B dogs fed with lyophilized lettuce cells expressing CTB-FIX. No side effects were detected after feeding CTB-FIX-lyophilized plant cells for >300 days. Coagulation times were markedly shortened by intravenous FIX in orally tolerized treated dogs, in contrast to control dogs that formed high-titer antibodies to FIX. Commercial-scale production, stability, prolonged storage of lyophilized cells, and efficacy in tolerance induction in a large, non-rodent model of human disease offer a novel concept for oral tolerance and low-cost production and delivery of biopharmaceuticals.
Keywords: factor IX; hemophilia; inhibitor; oral tolerance; transgenic plant.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


Comment in
-
Oral Tolerance: Another Reason to Eat Your Veggies!Mol Ther. 2017 Feb 1;25(2):311-313. doi: 10.1016/j.ymthe.2017.01.001. Epub 2017 Jan 18. Mol Ther. 2017. PMID: 28109961 Free PMC article. No abstract available.
Similar articles
-
Preclinical development of plant-based oral immune modulatory therapy for haemophilia B.Plant Biotechnol J. 2021 Oct;19(10):1952-1966. doi: 10.1111/pbi.13608. Epub 2021 May 15. Plant Biotechnol J. 2021. PMID: 33949086 Free PMC article.
-
Low cost industrial production of coagulation factor IX bioencapsulated in lettuce cells for oral tolerance induction in hemophilia B.Biomaterials. 2015 Nov;70:84-93. doi: 10.1016/j.biomaterials.2015.08.004. Epub 2015 Aug 5. Biomaterials. 2015. PMID: 26302233 Free PMC article.
-
Role of Small Intestine and Gut Microbiome in Plant-Based Oral Tolerance for Hemophilia.Front Immunol. 2020 May 20;11:844. doi: 10.3389/fimmu.2020.00844. eCollection 2020. Front Immunol. 2020. PMID: 32508814 Free PMC article.
-
Factor IX antibody and immune tolerance.Vox Sang. 1999;77 Suppl 1:70-1. doi: 10.1159/000056721. Vox Sang. 1999. PMID: 10529693 Review.
-
Tolerance induction in hemophilia: innovation and accomplishments.Curr Opin Hematol. 2018 Sep;25(5):365-372. doi: 10.1097/MOH.0000000000000446. Curr Opin Hematol. 2018. PMID: 29994897 Free PMC article. Review.
Cited by
-
Preclinical development of plant-based oral immune modulatory therapy for haemophilia B.Plant Biotechnol J. 2021 Oct;19(10):1952-1966. doi: 10.1111/pbi.13608. Epub 2021 May 15. Plant Biotechnol J. 2021. PMID: 33949086 Free PMC article.
-
Oral delivery of novel human IGF-1 bioencapsulated in lettuce cells promotes musculoskeletal cell proliferation, differentiation and diabetic fracture healing.Biomaterials. 2020 Mar;233:119591. doi: 10.1016/j.biomaterials.2019.119591. Epub 2019 Nov 5. Biomaterials. 2020. PMID: 31870566 Free PMC article.
-
The use of Bacillus subtilis as a cost-effective expression system for production of Cholera Toxin B fused factor VIII epitope regions applicable for inducing oral immune tolerance.Folia Microbiol (Praha). 2024 Dec;69(6):1267-1277. doi: 10.1007/s12223-024-01166-z. Epub 2024 Apr 29. Folia Microbiol (Praha). 2024. PMID: 38683262
-
Cas9-directed immune tolerance in humans-a model to evaluate regulatory T cells in gene therapy?Gene Ther. 2021 Sep;28(9):549-559. doi: 10.1038/s41434-021-00232-2. Epub 2021 Feb 11. Gene Ther. 2021. PMID: 33574580 Free PMC article. Review.
-
Cold chain and virus-free oral polio booster vaccine made in lettuce chloroplasts confers protection against all three poliovirus serotypes.Plant Biotechnol J. 2019 Jul;17(7):1357-1368. doi: 10.1111/pbi.13060. Epub 2019 Jan 11. Plant Biotechnol J. 2019. PMID: 30575284 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

