Propagation of pathological α-synuclein in marmoset brain
- PMID: 28148299
- PMCID: PMC5289012
- DOI: 10.1186/s40478-017-0413-0
Propagation of pathological α-synuclein in marmoset brain
Abstract
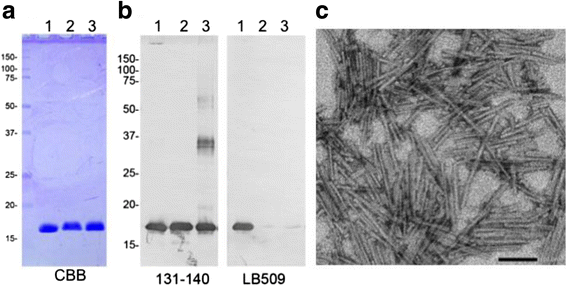
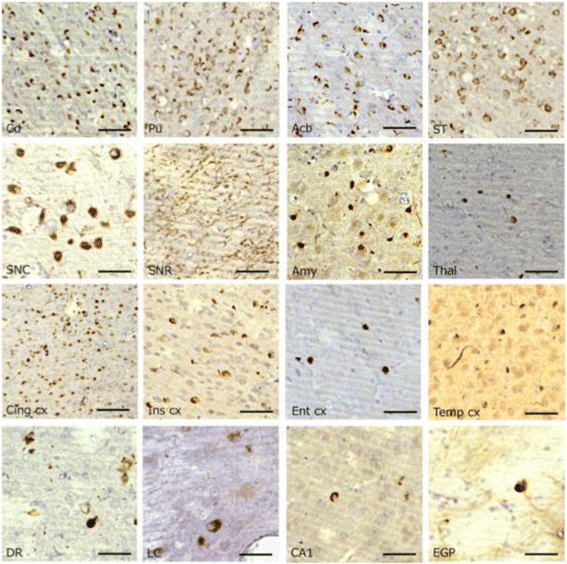
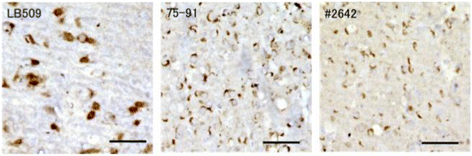
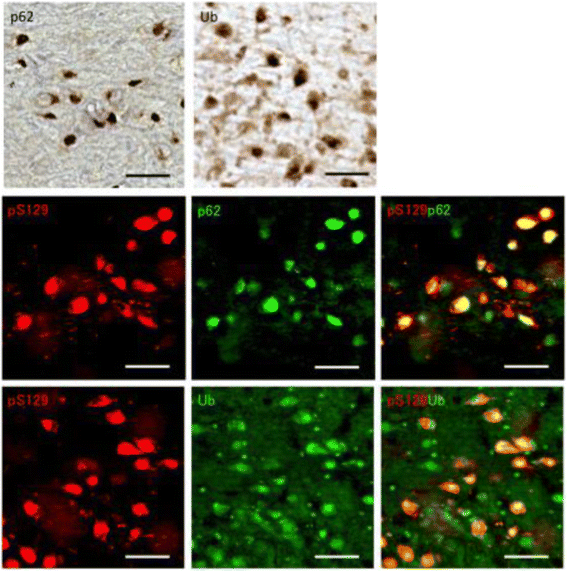
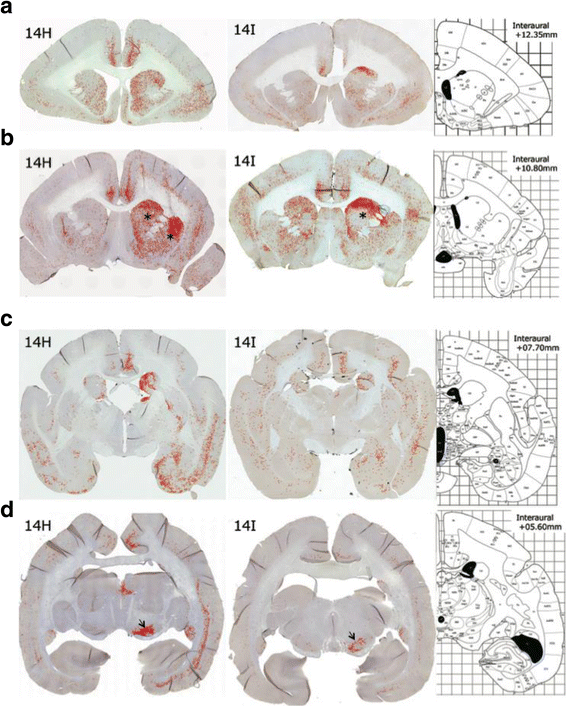
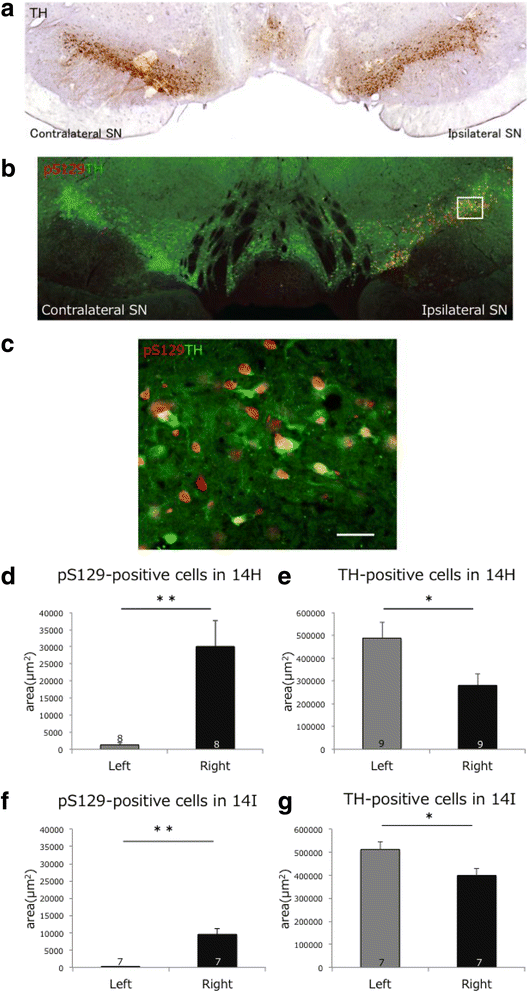
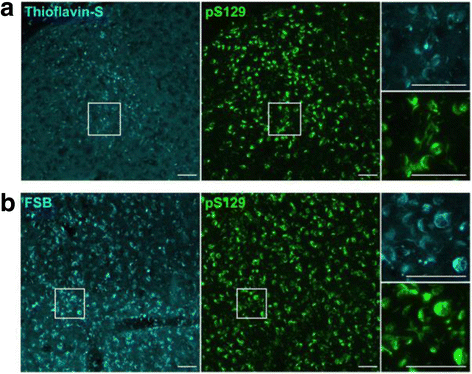
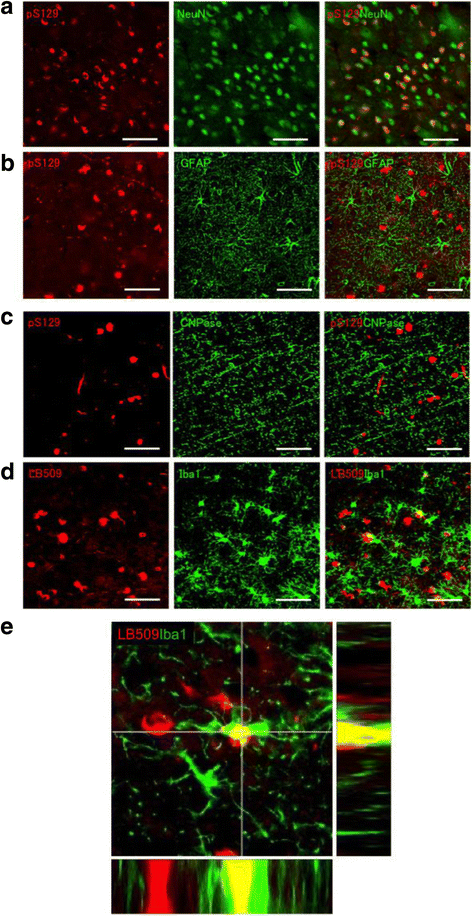
α-Synuclein is a defining, key component of Lewy bodies and Lewy neurites in Parkinson's disease (PD) and dementia with Lewy bodies (DLB), as well as glial cytoplasmic inclusions in multiple system atrophy (MSA). The distribution and spreading of these pathologies are closely correlated with disease progression. Recent studies have revealed that intracerebral injection of synthetic α-synuclein fibrils or pathological α-synuclein prepared from DLB or MSA brains into wild-type or transgenic animal brains induced prion-like propagation of phosphorylated α-synuclein pathology. The common marmoset is a very small primate that is expected to be a useful model of human diseases. Here, we show that intracerebral injection of synthetic α-synuclein fibrils into adult wild-type marmoset brains (caudate nucleus and/or putamen) resulted in spreading of abundant α-synuclein pathologies, which were positive for various antibodies to α-synuclein, including phospho Ser129-specific antibody, anti-ubiquitin and anti-p62 antibodies, at three months after injection. Remarkably, robust Lewy body-like inclusions were formed in tyrosine hydroxylase (TH)-positive neurons in these marmosets, strongly suggesting the retrograde spreading of abnormal α-synuclein from striatum to substantia nigra. Moreover, a significant decrease in the numbers of TH-positive neurons was observed in the injection-side of the brain, where α-synuclein inclusions were deposited. Furthermore, most of the α-synuclein inclusions were positive for 1-fluoro-2,5-bis (3-carboxy-4-hydroxystyryl) benzene (FSB) and thioflavin-S, which are dyes widely used to visualize the presence of amyloid. Thus, injection of synthetic α-synuclein fibrils into brains of non-transgenic primates induced PD-like α-synuclein pathologies within only 3 months after injection. Finally, we provide evidence indicating that neurons with abnormal α-synuclein inclusions may be cleared by microglial cells. This is the first marmoset model for α-synuclein propagation. It should be helpful in studies to elucidate mechanisms of disease progression and in development and evaluation of disease-modifying drugs for α-synucleinopathies.
Keywords: Circuits; Marmoset; Parkinson; Prion; α-synuclein.
Figures

Similar articles
-
AAV1/2-induced overexpression of A53T-α-synuclein in the substantia nigra results in degeneration of the nigrostriatal system with Lewy-like pathology and motor impairment: a new mouse model for Parkinson's disease.Acta Neuropathol Commun. 2017 Feb 1;5(1):11. doi: 10.1186/s40478-017-0416-x. Acta Neuropathol Commun. 2017. PMID: 28143577 Free PMC article.
-
Intrastriatal alpha-synuclein fibrils in monkeys: spreading, imaging and neuropathological changes.Brain. 2019 Nov 1;142(11):3565-3579. doi: 10.1093/brain/awz296. Brain. 2019. PMID: 31580415 Free PMC article.
-
[Prion-like Propagation of Pathological α-Synuclein in Vivo].Yakugaku Zasshi. 2019;139(7):1007-1013. doi: 10.1248/yakushi.18-00165-4. Yakugaku Zasshi. 2019. PMID: 31257247 Review. Japanese.
-
Progression of phosphorylated α-synuclein in Macaca fuscata.Brain Pathol. 2021 Sep;31(5):e12952. doi: 10.1111/bpa.12952. Epub 2021 Mar 22. Brain Pathol. 2021. PMID: 33754430 Free PMC article.
-
Is Multiple System Atrophy a Prion-like Disorder?Int J Mol Sci. 2021 Sep 18;22(18):10093. doi: 10.3390/ijms221810093. Int J Mol Sci. 2021. PMID: 34576255 Free PMC article. Review.
Cited by
-
Selecting the Best Animal Model of Parkinson's Disease for Your Research Purpose: Insight from in vivo PET Imaging Studies.Curr Neuropharmacol. 2023;21(5):1241-1272. doi: 10.2174/1570159X21666230216101659. Curr Neuropharmacol. 2023. PMID: 36797611 Free PMC article.
-
The basis of clinicopathological heterogeneity in TDP-43 proteinopathy.Acta Neuropathol. 2019 Nov;138(5):751-770. doi: 10.1007/s00401-019-02077-x. Epub 2019 Sep 26. Acta Neuropathol. 2019. PMID: 31555895 Free PMC article. Review.
-
Anatomical variability, multi-modal coordinate systems, and precision targeting in the marmoset brain.Neuroimage. 2022 Apr 15;250:118965. doi: 10.1016/j.neuroimage.2022.118965. Epub 2022 Feb 2. Neuroimage. 2022. PMID: 35122965 Free PMC article.
-
An in vivo Pig Model for Testing Novel Positron Emission Tomography Radioligands Targeting Cerebral Protein Aggregates.Front Neurosci. 2022 Mar 16;16:847074. doi: 10.3389/fnins.2022.847074. eCollection 2022. Front Neurosci. 2022. PMID: 35368260 Free PMC article.
-
Short chain fatty acids-producing and mucin-degrading intestinal bacteria predict the progression of early Parkinson's disease.NPJ Parkinsons Dis. 2022 Jun 1;8(1):65. doi: 10.1038/s41531-022-00328-5. NPJ Parkinsons Dis. 2022. PMID: 35650236 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

