Formative pluripotency: the executive phase in a developmental continuum
- PMID: 28143843
- PMCID: PMC5430734
- DOI: 10.1242/dev.142679
Formative pluripotency: the executive phase in a developmental continuum
Abstract
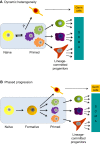
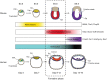
The regulative capability of single cells to give rise to all primary embryonic lineages is termed pluripotency. Observations of fluctuating gene expression and phenotypic heterogeneity in vitro have fostered a conception of pluripotency as an intrinsically metastable and precarious state. However, in the embryo and in defined culture environments the properties of pluripotent cells change in an orderly sequence. Two phases of pluripotency, called naïve and primed, have previously been described. In this Hypothesis article, a third phase, called formative pluripotency, is proposed to exist as part of a developmental continuum between the naïve and primed phases. The formative phase is hypothesised to be enabling for the execution of pluripotency, entailing remodelling of transcriptional, epigenetic, signalling and metabolic networks to constitute multi-lineage competence and responsiveness to specification cues.
Keywords: Developmental potential; Embryonic stem cells; Epiblast; Lineage specification; Pluripotency.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
The author declares no competing or financial interests.
Figures
Similar articles
-
Mapping the route from naive pluripotency to lineage specification.Philos Trans R Soc Lond B Biol Sci. 2014 Dec 5;369(1657):20130540. doi: 10.1098/rstb.2013.0540. Philos Trans R Soc Lond B Biol Sci. 2014. PMID: 25349449 Free PMC article. Review.
-
Lineage-Specific Profiling Delineates the Emergence and Progression of Naive Pluripotency in Mammalian Embryogenesis.Dev Cell. 2015 Nov 9;35(3):366-82. doi: 10.1016/j.devcel.2015.10.011. Dev Cell. 2015. PMID: 26555056 Free PMC article.
-
Multi-omic Profiling Reveals Dynamics of the Phased Progression of Pluripotency.Cell Syst. 2019 May 22;8(5):427-445.e10. doi: 10.1016/j.cels.2019.03.012. Epub 2019 May 8. Cell Syst. 2019. PMID: 31078527 Free PMC article.
-
Capturing Pluripotency and Beyond.Cells. 2021 Dec 16;10(12):3558. doi: 10.3390/cells10123558. Cells. 2021. PMID: 34944066 Free PMC article. Review.
-
Mechanisms of pluripotency in vivo and in vitro.Curr Top Dev Biol. 2014;107:1-37. doi: 10.1016/B978-0-12-416022-4.00001-9. Curr Top Dev Biol. 2014. PMID: 24439801 Review.
Cited by
-
Human ES Cell Culture Conditions Fail to Preserve the Mouse Epiblast State.Stem Cells Int. 2021 Mar 10;2021:8818356. doi: 10.1155/2021/8818356. eCollection 2021. Stem Cells Int. 2021. PMID: 33828592 Free PMC article.
-
Evidence of Immunoproteasome Expression Onset in the Formative State of Pluripotency in Mouse Cells.Cells. 2024 Aug 15;13(16):1362. doi: 10.3390/cells13161362. Cells. 2024. PMID: 39195252 Free PMC article.
-
Resistance to Naïve and Formative Pluripotency Conversion in RSeT Human Embryonic Stem Cells.bioRxiv [Preprint]. 2024 Apr 12:2024.02.16.580778. doi: 10.1101/2024.02.16.580778. bioRxiv. 2024. PMID: 38410444 Free PMC article. Preprint.
-
DNA methylation restricts coordinated germline and neural fates in embryonic stem cell differentiation.Nat Struct Mol Biol. 2024 Jan;31(1):102-114. doi: 10.1038/s41594-023-01162-w. Epub 2024 Jan 4. Nat Struct Mol Biol. 2024. PMID: 38177678
-
Specification of the First Mammalian Cell Lineages In Vivo and In Vitro.Cold Spring Harb Perspect Biol. 2020 Apr 1;12(4):a035634. doi: 10.1101/cshperspect.a035634. Cold Spring Harb Perspect Biol. 2020. PMID: 31615786 Free PMC article. Review.
References
-
- Acampora D., Omodei D., Petrosino G., Garofalo A., Savarese M., Nigro V., Di Giovannantonio L. G., Mercadante V. and Simeone A. (2016). Loss of the Otx2-binding site in the nanog promoter affects the integrity of embryonic stem cell subtypes and specification of inner cell mass-derived epiblast. Cell Rep. 15, 2651-2664. 10.1016/j.celrep.2016.05.041 - DOI - PubMed
-
- Beddington R. S. (1983). Histogenetic and neoplastic potential of different regions of the mouse embryonic egg cylinder. J. Embryol. Exp. Morphol. 75, 189-204. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

