Anifrolumab, an Anti-Interferon-α Receptor Monoclonal Antibody, in Moderate-to-Severe Systemic Lupus Erythematosus
- PMID: 28130918
- PMCID: PMC5299497
- DOI: 10.1002/art.39962
Anifrolumab, an Anti-Interferon-α Receptor Monoclonal Antibody, in Moderate-to-Severe Systemic Lupus Erythematosus
Abstract
Objective: To assess the efficacy and safety of anifrolumab, a type I interferon (IFN) receptor antagonist, in a phase IIb, randomized, double-blind, placebo-controlled study of adults with moderate-to-severe systemic lupus erythematosus (SLE).
Methods: Patients (n = 305) were randomized to receive intravenous anifrolumab (300 mg or 1,000 mg) or placebo, in addition to standard therapy, every 4 weeks for 48 weeks. Randomization was stratified by SLE Disease Activity Index 2000 score (<10 or ≥10), oral corticosteroid dosage (<10 or ≥10 mg/day), and type I IFN gene signature test status (high or low) based on a 4-gene expression assay. The primary end point was the percentage of patients achieving an SLE Responder Index (SRI[4]) response at week 24 with sustained reduction of oral corticosteroids (<10 mg/day and less than or equal to the dose at week 1 from week 12 through 24). Other end points (including SRI[4], British Isles Lupus Assessment Group [BILAG]-based Composite Lupus Assessment [BICLA], modified SRI[6], and major clinical response) were assessed at week 52. The primary end point was analyzed in the modified intent-to-treat (ITT) population and type I IFN-high subpopulation. The study result was considered positive if the primary end point was met in either of the 2 study populations. The Type I error rate was controlled at 0.10 (2-sided), within each of the 2 study populations for the primary end point analysis.
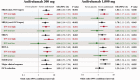
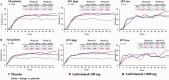
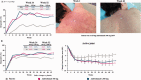
Results: The primary end point was met by more patients treated with anifrolumab (34.3% of 99 for 300 mg and 28.8% of 104 for 1,000 mg) than placebo (17.6% of 102) (P = 0.014 for 300 mg and P = 0.063 for 1,000 mg, versus placebo), with greater effect size in patients with a high IFN signature at baseline (13.2% in placebo-treated patients versus 36.0% [P = 0.004] and 28.2% [P = 0.029]) in patients treated with anifrolumab 300 mg and 1,000 mg, respectively. At week 52, patients treated with anifrolumab achieved greater responses in SRI(4) (40.2% versus 62.6% [P < 0.001] and 53.8% [P = 0.043] with placebo, anifrolumab 300 mg, and anifrolumab 1,000 mg, respectively), BICLA (25.7% versus 53.5% [P < 0.001] and 41.2% [P = 0.018], respectively), modified SRI(6) (28.4% versus 49.5% [P = 0.002] and 44.7% [P = 0.015], respectively), major clinical response (BILAG 2004 C or better in all organ domains from week 24 through week 52) (6.9% versus 19.2% [P = 0.012] and 17.3% [P = 0.025], respectively), and several other global and organ-specific end points. Herpes zoster was more frequent in the anifrolumab-treated patients (2.0% with placebo treatment versus 5.1% and 9.5% with anifrolumab 300 mg and 1,000 mg, respectively), as were cases reported as influenza (2.0% versus 6.1% and 7.6%, respectively), in the anifrolumab treatment groups. Incidence of serious adverse events was similar between groups (18.8% versus 16.2% and 17.1%, respectively).
Conclusion: Anifrolumab substantially reduced disease activity compared with placebo across multiple clinical end points in the patients with moderate-to-severe SLE.
Trial registration: ClinicalTrials.gov NCT01438489.
© 2016 The Authors. Arthritis & Rheumatology published by Wiley Periodicals, Inc. on behalf of the American College of Rheumatology.
Figures
Comment in
-
Editorial: Interferon-Targeted Therapy for Systemic Lupus Erythematosus: Are the Trials on Target?Arthritis Rheumatol. 2017 Feb;69(2):245-248. doi: 10.1002/art.39985. Arthritis Rheumatol. 2017. PMID: 27860434 No abstract available.
Similar articles
-
Interferon Inhibition for Lupus with Anifrolumab: Critical Appraisal of the Evidence Leading to FDA Approval.ACR Open Rheumatol. 2022 Jun;4(6):486-491. doi: 10.1002/acr2.11414. Epub 2022 Feb 14. ACR Open Rheumatol. 2022. PMID: 35157371 Free PMC article.
-
Trial of Anifrolumab in Active Systemic Lupus Erythematosus.N Engl J Med. 2020 Jan 16;382(3):211-221. doi: 10.1056/NEJMoa1912196. Epub 2019 Dec 18. N Engl J Med. 2020. PMID: 31851795 Clinical Trial.
-
Type I interferon inhibitor anifrolumab in active systemic lupus erythematosus (TULIP-1): a randomised, controlled, phase 3 trial.Lancet Rheumatol. 2019 Dec;1(4):e208-e219. doi: 10.1016/S2665-9913(19)30076-1. Epub 2019 Nov 11. Lancet Rheumatol. 2019. PMID: 38229377
-
Anifrolumab, a monoclonal antibody to the type I interferon receptor subunit 1, for the treatment of systemic lupus erythematosus: an overview from clinical trials.Mod Rheumatol. 2021 Jan;31(1):1-12. doi: 10.1080/14397595.2020.1812201. Epub 2020 Sep 17. Mod Rheumatol. 2021. PMID: 32814461 Review.
-
Spotlight on anifrolumab and its potential for the treatment of moderate-to-severe systemic lupus erythematosus: evidence to date.Drug Des Devel Ther. 2019 May 8;13:1535-1543. doi: 10.2147/DDDT.S170969. eCollection 2019. Drug Des Devel Ther. 2019. PMID: 31190735 Free PMC article. Review.
Cited by
-
Centrally Acting Angiotensin-Converting Enzyme Inhibitor Suppresses Type I Interferon Responses and Decreases Inflammation in the Periphery and the CNS in Lupus-Prone Mice.Front Immunol. 2020 Sep 15;11:573677. doi: 10.3389/fimmu.2020.573677. eCollection 2020. Front Immunol. 2020. PMID: 33042154 Free PMC article.
-
Modulation of Cardiometabolic Disease Markers by Type I Interferon Inhibition in Systemic Lupus Erythematosus.Arthritis Rheumatol. 2021 Mar;73(3):459-471. doi: 10.1002/art.41518. Epub 2021 Feb 15. Arthritis Rheumatol. 2021. PMID: 32909675 Free PMC article. Clinical Trial.
-
Emerging concepts of type I interferons in SLE pathogenesis and therapy.Nat Rev Rheumatol. 2022 Oct;18(10):575-590. doi: 10.1038/s41584-022-00826-z. Epub 2022 Sep 12. Nat Rev Rheumatol. 2022. PMID: 36097207 Review.
-
Type I Interferon Production of Plasmacytoid Dendritic Cells under Control.Int J Mol Sci. 2021 Apr 18;22(8):4190. doi: 10.3390/ijms22084190. Int J Mol Sci. 2021. PMID: 33919546 Free PMC article. Review.
-
Role of the Innate Immunity Signaling Pathway in the Pathogenesis of Sjögren's Syndrome.Int J Mol Sci. 2021 Mar 17;22(6):3090. doi: 10.3390/ijms22063090. Int J Mol Sci. 2021. PMID: 33803026 Free PMC article. Review.
References
-
- Mathian A, Hie M, Cohen‐Aubart F, Amoura Z. Targeting interferons in systemic lupus erythematosus: current and future prospects. Drugs 2015;75:835–46. - PubMed
-
- Bernatsky S, Boivin JF, Joseph L, Manzi S, Ginzler E, Gladman DD, et al. Mortality in systemic lupus erythematosus. Arthritis Rheum 2006;54:2550–7. - PubMed
-
- Panopalis P, Clarke AE, Yelin E. The economic burden of systemic lupus erythematosus. Best Pract Res Clin Rheumatol 2012;26:695–704. - PubMed
-
- Yurkovich M, Vostretsova K, Chen W, Aviña‐Zubieta JA. Overall and cause‐specific mortality in patients with systemic lupus erythematosus: a meta‐analysis of observational studies. Arthritis Care Res (Hoboken) 2014;66:608–16. - PubMed
-
- Petri M. Long‐term outcomes in lupus. Am J Manag Care 2001;7 Suppl:S480–5. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

