Tissue reservoirs of antiviral T cell immunity in persistent human CMV infection
- PMID: 28130404
- PMCID: PMC5339671
- DOI: 10.1084/jem.20160758
Tissue reservoirs of antiviral T cell immunity in persistent human CMV infection
Abstract
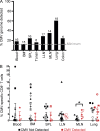
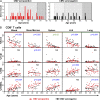
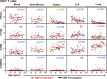
T cell responses to viruses are initiated and maintained in tissue sites; however, knowledge of human antiviral T cells is largely derived from blood. Cytomegalovirus (CMV) persists in most humans, requires T cell immunity to control, yet tissue immune responses remain undefined. Here, we investigated human CMV-specific T cells, virus persistence and CMV-associated T cell homeostasis in blood, lymphoid, mucosal and secretory tissues of 44 CMV seropositive and 28 seronegative donors. CMV-specific T cells were maintained in distinct distribution patterns, highest in blood, bone marrow (BM), or lymph nodes (LN), with the frequency and function in blood distinct from tissues. CMV genomes were detected predominantly in lung and also in spleen, BM, blood and LN. High frequencies of activated CMV-specific T cells were found in blood and BM samples with low virus detection, whereas in lung, CMV-specific T cells were present along with detectable virus. In LNs, CMV-specific T cells exhibited quiescent phenotypes independent of virus. Overall, T cell differentiation was enhanced in sites of viral persistence with age. Together, our results suggest tissue T cell reservoirs for CMV control shaped by both viral and tissue-intrinsic factors, with global effects on homeostasis of tissue T cells over the lifespan.
@Gordon et al.
Figures




Similar articles
-
Distribution and functional analysis of memory antiviral CD8 T cell responses in HIV-1 and cytomegalovirus infections.Eur J Immunol. 2002 Dec;32(12):3756-64. doi: 10.1002/1521-4141(200212)32:12<3756::AID-IMMU3756>3.0.CO;2-E. Eur J Immunol. 2002. PMID: 12516570
-
[Monitoring of cytomegalovirus-specific CD4+ and CD8+ T cell responses by cytokine flow cytometry in renal transplant recipients].Mikrobiyol Bul. 2016 Apr;50(2):224-35. Mikrobiyol Bul. 2016. PMID: 27175495 Turkish.
-
Dominance of virus-specific CD8 T cells in human primary cytomegalovirus infection.J Am Soc Nephrol. 2002 Oct;13(10):2577-84. doi: 10.1097/01.asn.0000030141.41726.52. J Am Soc Nephrol. 2002. PMID: 12239248
-
Analyzing T-cell responses to cytomegalovirus by cytokine flow cytometry.Hum Immunol. 2004 May;65(5):493-9. doi: 10.1016/j.humimm.2004.02.004. Hum Immunol. 2004. PMID: 15172449 Review.
-
Control of Cytomegalovirus Viremia after Allogeneic Stem Cell Transplantation: A Review on CMV-Specific T Cell Reconstitution.Biol Blood Marrow Transplant. 2018 Sep;24(9):1776-1782. doi: 10.1016/j.bbmt.2018.03.028. Epub 2018 Apr 4. Biol Blood Marrow Transplant. 2018. PMID: 29626514 Review.
Cited by
-
Breadth and polyfunctionality of T cell responses to human cytomegalovirus in men who have sex with men: relationship with HIV infection and frailty.J Virol. 2024 Oct 22;98(10):e0116724. doi: 10.1128/jvi.01167-24. Epub 2024 Sep 4. J Virol. 2024. PMID: 39230302
-
Immunophenotyping of Rhesus CMV-Specific CD8 T-Cell Populations.Cytometry A. 2021 Mar;99(3):278-288. doi: 10.1002/cyto.a.24197. Epub 2020 Aug 4. Cytometry A. 2021. PMID: 32713108 Free PMC article.
-
Tissue-Resident Immune Cells in Humans.Annu Rev Immunol. 2022 Apr 26;40:195-220. doi: 10.1146/annurev-immunol-093019-112809. Epub 2022 Jan 19. Annu Rev Immunol. 2022. PMID: 35044795 Free PMC article. Review.
-
Newborn and child-like molecular signatures in older adults stem from TCR shifts across human lifespan.Nat Immunol. 2023 Nov;24(11):1890-1907. doi: 10.1038/s41590-023-01633-8. Epub 2023 Sep 25. Nat Immunol. 2023. PMID: 37749325 Free PMC article.
-
Erratum: Systemic Characterization of Novel Immune Cell Phenotypes in Recurrent Pregnancy Loss.Front Immunol. 2021 Jun 23;12:722805. doi: 10.3389/fimmu.2021.722805. eCollection 2021. Front Immunol. 2021. PMID: 34249023 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

