P3h3-null and Sc65-null Mice Phenocopy the Collagen Lysine Under-hydroxylation and Cross-linking Abnormality of Ehlers-Danlos Syndrome Type VIA
- PMID: 28115524
- PMCID: PMC5339768
- DOI: 10.1074/jbc.M116.762245
P3h3-null and Sc65-null Mice Phenocopy the Collagen Lysine Under-hydroxylation and Cross-linking Abnormality of Ehlers-Danlos Syndrome Type VIA
Abstract
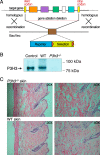
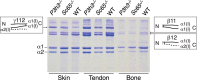
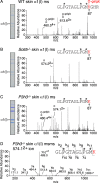
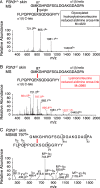
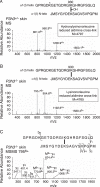
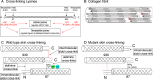
Tandem mass spectrometry was applied to tissues from targeted mutant mouse models to explore the collagen substrate specificities of individual members of the prolyl 3-hydroxylase (P3H) gene family. Previous studies revealed that P3h1 preferentially 3-hydroxylates proline at a single site in collagen type I chains, whereas P3h2 is responsible for 3-hydroxylating multiple proline sites in collagen types I, II, IV, and V. In screening for collagen substrate sites for the remaining members of the vertebrate P3H family, P3h3 and Sc65 knock-out mice revealed a common lysine under-hydroxylation effect at helical domain cross-linking sites in skin, bone, tendon, aorta, and cornea. No effect on prolyl 3-hydroxylation was evident on screening the spectrum of known 3-hydroxyproline sites from all major tissue collagen types. However, collagen type I extracted from both Sc65-/- and P3h3-/- skin revealed the same abnormal chain pattern on SDS-PAGE with an overabundance of a γ112 cross-linked trimer. The latter proved to be from native molecules that had intramolecular aldol cross-links at each end. The lysine under-hydroxylation was shown to alter the divalent aldimine cross-link chemistry of mutant skin collagen. Furthermore, the ratio of mature HP/LP cross-links in bone of both P3h3-/- and Sc65-/- mice was reversed compared with wild type, consistent with the level of lysine under-hydroxylation seen in individual chains at cross-linking sites. The effect on cross-linking lysines was quantitatively very similar to that previously observed in EDS VIA human and Plod1-/- mouse tissues, suggesting that P3H3 and/or SC65 mutations may cause as yet undefined EDS variants.
Keywords: bone; collagen; cross-links; endoplasmic reticulum (ER); mass spectrometry (MS); post-translational modification (PTM); skin.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


Similar articles
-
Post-translationally abnormal collagens of prolyl 3-hydroxylase-2 null mice offer a pathobiological mechanism for the high myopia linked to human LEPREL1 mutations.J Biol Chem. 2015 Mar 27;290(13):8613-22. doi: 10.1074/jbc.M114.634915. Epub 2015 Feb 2. J Biol Chem. 2015. PMID: 25645914 Free PMC article.
-
Type I and type V procollagen triple helix uses different subsets of the molecular ensemble for lysine posttranslational modifications in the rER.J Biol Chem. 2021 Jan-Jun;296:100453. doi: 10.1016/j.jbc.2021.100453. Epub 2021 Feb 23. J Biol Chem. 2021. PMID: 33631195 Free PMC article.
-
Sc65-Null Mice Provide Evidence for a Novel Endoplasmic Reticulum Complex Regulating Collagen Lysyl Hydroxylation.PLoS Genet. 2016 Apr 27;12(4):e1006002. doi: 10.1371/journal.pgen.1006002. eCollection 2016 Apr. PLoS Genet. 2016. PMID: 27119146 Free PMC article.
-
Collagen prolyl 3-hydroxylation: a major role for a minor post-translational modification?Connect Tissue Res. 2013;54(4-5):245-51. doi: 10.3109/03008207.2013.800867. Epub 2013 Jun 21. Connect Tissue Res. 2013. PMID: 23772978 Free PMC article. Review.
-
Defects in the biochemistry of collagen in diseases of connective tissue.J Invest Dermatol. 1976 Feb;66(02):59-79. doi: 10.1111/1523-1747.ep12481404. J Invest Dermatol. 1976. PMID: 1448 Review.
Cited by
-
Collagen molecular phenotypic switch between non-neoplastic and neoplastic canine mammary tissues.Sci Rep. 2021 Apr 21;11(1):8659. doi: 10.1038/s41598-021-87380-y. Sci Rep. 2021. PMID: 33883562 Free PMC article.
-
Quantitative proteomic profiling of extracellular matrix and site-specific collagen post-translational modifications in an in vitro model of lung fibrosis.Matrix Biol Plus. 2019 Apr 13;1:100005. doi: 10.1016/j.mbplus.2019.04.002. eCollection 2019 Feb. Matrix Biol Plus. 2019. PMID: 33543004 Free PMC article.
-
Substitution of murine type I collagen A1 3-hydroxylation site alters matrix structure but does not recapitulate osteogenesis imperfecta bone dysplasia.Matrix Biol. 2020 Aug;90:20-39. doi: 10.1016/j.matbio.2020.02.003. Epub 2020 Feb 26. Matrix Biol. 2020. PMID: 32112888 Free PMC article.
-
Role of prolyl hydroxylation in the molecular interactions of collagens.Essays Biochem. 2019 Sep 13;63(3):325-335. doi: 10.1042/EBC20180053. Print 2019 Sep 13. Essays Biochem. 2019. PMID: 31350381 Free PMC article. Review.
-
The triple helix of collagens - an ancient protein structure that enabled animal multicellularity and tissue evolution.J Cell Sci. 2018 Apr 9;131(7):jcs203950. doi: 10.1242/jcs.203950. J Cell Sci. 2018. PMID: 29632050 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

