ACBD5 and VAPB mediate membrane associations between peroxisomes and the ER
- PMID: 28108524
- PMCID: PMC5294785
- DOI: 10.1083/jcb.201607055
ACBD5 and VAPB mediate membrane associations between peroxisomes and the ER
Abstract
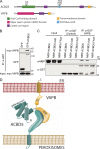
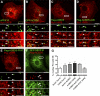
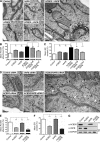
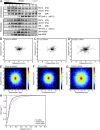
Peroxisomes (POs) and the endoplasmic reticulum (ER) cooperate in cellular lipid metabolism and form tight structural associations, which were first observed in ultrastructural studies decades ago. PO-ER associations have been suggested to impact on a diverse number of physiological processes, including lipid metabolism, phospholipid exchange, metabolite transport, signaling, and PO biogenesis. Despite their fundamental importance to cell metabolism, the mechanisms by which regions of the ER become tethered to POs are unknown, in particular in mammalian cells. Here, we identify the PO membrane protein acyl-coenzyme A-binding domain protein 5 (ACBD5) as a binding partner for the resident ER protein vesicle-associated membrane protein-associated protein B (VAPB). We show that ACBD5-VAPB interaction regulates PO-ER associations. Moreover, we demonstrate that loss of PO-ER association perturbs PO membrane expansion and increases PO movement. Our findings reveal the first molecular mechanism for establishing PO-ER associations in mammalian cells and report a new function for ACBD5 in PO-ER tethering.
© 2017 Costello et al.
Figures
Comment in
-
Incredibly close-A newly identified peroxisome-ER contact site in humans.J Cell Biol. 2017 Feb;216(2):287-289. doi: 10.1083/jcb.201701072. Epub 2017 Jan 20. J Cell Biol. 2017. PMID: 28108527 Free PMC article.
-
Organelle dynamics: Connections, connections, connections.Nat Rev Mol Cell Biol. 2017 Feb 21;18(3):139. doi: 10.1038/nrm.2017.14. Nat Rev Mol Cell Biol. 2017. PMID: 28220048 No abstract available.
Similar articles
-
Intracellular redistribution of neuronal peroxisomes in response to ACBD5 expression.PLoS One. 2018 Dec 27;13(12):e0209507. doi: 10.1371/journal.pone.0209507. eCollection 2018. PLoS One. 2018. PMID: 30589881 Free PMC article.
-
VAPs and ACBD5 tether peroxisomes to the ER for peroxisome maintenance and lipid homeostasis.J Cell Biol. 2017 Feb;216(2):367-377. doi: 10.1083/jcb.201608128. Epub 2017 Jan 20. J Cell Biol. 2017. PMID: 28108526 Free PMC article.
-
Regulating peroxisome-ER contacts via the ACBD5-VAPB tether by FFAT motif phosphorylation and GSK3β.J Cell Biol. 2022 Mar 7;221(3):e202003143. doi: 10.1083/jcb.202003143. Epub 2022 Jan 12. J Cell Biol. 2022. PMID: 35019937 Free PMC article.
-
Peroxisomes: membrane events accompanying peroxisome proliferation.Int J Biochem Cell Biol. 2011 Jun;43(6):847-51. doi: 10.1016/j.biocel.2011.03.006. Epub 2011 Mar 17. Int J Biochem Cell Biol. 2011. PMID: 21419861 Review.
-
Organelle interplay-peroxisome interactions in health and disease.J Inherit Metab Dis. 2020 Jan;43(1):71-89. doi: 10.1002/jimd.12083. Epub 2019 Apr 16. J Inherit Metab Dis. 2020. PMID: 30864148 Free PMC article. Review.
Cited by
-
Intracellular redistribution of neuronal peroxisomes in response to ACBD5 expression.PLoS One. 2018 Dec 27;13(12):e0209507. doi: 10.1371/journal.pone.0209507. eCollection 2018. PLoS One. 2018. PMID: 30589881 Free PMC article.
-
Sequestosome 1 Is Part of the Interaction Network of VAPB.Int J Mol Sci. 2021 Dec 9;22(24):13271. doi: 10.3390/ijms222413271. Int J Mol Sci. 2021. PMID: 34948065 Free PMC article.
-
Peroxisomal targeting of a protein phosphatase type 2C via mitochondrial transit.Nat Commun. 2020 May 12;11(1):2355. doi: 10.1038/s41467-020-16146-3. Nat Commun. 2020. PMID: 32398688 Free PMC article.
-
Coming together to define membrane contact sites.Nat Commun. 2019 Mar 20;10(1):1287. doi: 10.1038/s41467-019-09253-3. Nat Commun. 2019. PMID: 30894536 Free PMC article. Review.
-
Redox crosstalk at endoplasmic reticulum (ER) membrane contact sites (MCS) uses toxic waste to deliver messages.Cell Death Dis. 2018 Feb 28;9(3):331. doi: 10.1038/s41419-017-0033-4. Cell Death Dis. 2018. PMID: 29491367 Free PMC article. Review.
References
-
- Abu-Safieh, L., Alrashed M., Anazi S., Alkuraya H., Khan A.O., Al-Owain M., Al-Zahrani J., Al-Abdi L., Hashem M., Al-Tarimi S., et al. . 2013. Autozygome-guided exome sequencing in retinal dystrophy patients reveals pathogenetic mutations and novel candidate disease genes. Genome Res. 23:236–247. 10.1101/gr.144105.112 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

