Biomarker-Based PET Imaging of Diffuse Intrinsic Pontine Glioma in Mouse Models
- PMID: 28108511
- PMCID: PMC5392368
- DOI: 10.1158/0008-5472.CAN-16-2850
Biomarker-Based PET Imaging of Diffuse Intrinsic Pontine Glioma in Mouse Models
Abstract
Diffuse intrinsic pontine glioma (DIPG) is a childhood brainstem tumor with a universally poor prognosis. Here, we characterize a positron emission tomography (PET) probe for imaging DIPG in vivo In human histological tissues, the probes target, PARP1, was highly expressed in DIPG compared to normal brain. PET imaging allowed for the sensitive detection of DIPG in a genetically engineered mouse model, and probe uptake correlated to histologically determined tumor infiltration. Imaging with the sister fluorescence agent revealed that uptake was confined to proliferating, PARP1-expressing cells. Comparison with other imaging technologies revealed remarkable accuracy of our biomarker approach. We subsequently demonstrated that serial imaging of DIPG in mouse models enables monitoring of tumor growth, as shown in modeling of tumor progression. Overall, this validated method for quantifying DIPG burden would serve useful in monitoring treatment response in early phase clinical trials. Cancer Res; 77(8); 2112-23. ©2017 AACR.
©2017 American Association for Cancer Research.
Conflict of interest statement
Figures
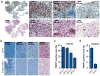
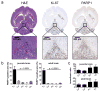
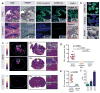
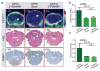
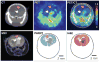
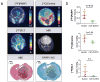
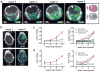
Comment in
-
Positron emission tomography imaging in diffuse intrinsic pontine glioma.Ann Transl Med. 2017 Aug;5(15):312. doi: 10.21037/atm.2017.05.02. Ann Transl Med. 2017. PMID: 28856152 Free PMC article. No abstract available.
Similar articles
-
Bevacizumab Targeting Diffuse Intrinsic Pontine Glioma: Results of 89Zr-Bevacizumab PET Imaging in Brain Tumor Models.Mol Cancer Ther. 2016 Sep;15(9):2166-74. doi: 10.1158/1535-7163.MCT-15-0558. Epub 2016 Jun 20. Mol Cancer Ther. 2016. PMID: 27325687
-
Evaluation of 11C-Methionine PET and Anatomic MRI Associations in Diffuse Intrinsic Pontine Glioma.J Nucl Med. 2019 Mar;60(3):312-319. doi: 10.2967/jnumed.118.212514. Epub 2018 Aug 2. J Nucl Med. 2019. PMID: 30072503 Free PMC article.
-
Molecular Drug Imaging: 89Zr-Bevacizumab PET in Children with Diffuse Intrinsic Pontine Glioma.J Nucl Med. 2017 May;58(5):711-716. doi: 10.2967/jnumed.116.180216. Epub 2016 Oct 20. J Nucl Med. 2017. PMID: 27765855 Clinical Trial.
-
Developing chemotherapy for diffuse pontine intrinsic gliomas (DIPG).Crit Rev Oncol Hematol. 2017 Dec;120:111-119. doi: 10.1016/j.critrevonc.2017.10.013. Epub 2017 Oct 31. Crit Rev Oncol Hematol. 2017. PMID: 29198324 Review.
-
Conventional and Advanced Imaging of Diffuse Intrinsic Pontine Glioma.J Child Neurol. 2016 Oct;31(12):1386-93. doi: 10.1177/0883073816634855. Epub 2016 Apr 12. J Child Neurol. 2016. PMID: 27071471 Free PMC article. Review.
Cited by
-
PET tracers in glioblastoma: Toward neurotheranostics as an individualized medicine approach.Front Nucl Med. 2023 Feb 27;3:1103262. doi: 10.3389/fnume.2023.1103262. eCollection 2023. Front Nucl Med. 2023. PMID: 39355049 Free PMC article. Review.
-
Discriminating radiation injury from recurrent tumor with [18F]PARPi and amino acid PET in mouse models.EJNMMI Res. 2018 Jul 4;8(1):59. doi: 10.1186/s13550-018-0399-z. EJNMMI Res. 2018. PMID: 29974335 Free PMC article.
-
Fluorescein-stained confocal laser endomicroscopy versus conventional frozen section for intraoperative histopathological assessment of intracranial tumors.Neuro Oncol. 2024 May 3;26(5):922-932. doi: 10.1093/neuonc/noae006. Neuro Oncol. 2024. PMID: 38243410 Free PMC article. Clinical Trial.
-
Poly(ADP-Ribose)Polymerase (PARP) Inhibitors and Radiation Therapy.Front Pharmacol. 2020 Mar 3;11:170. doi: 10.3389/fphar.2020.00170. eCollection 2020. Front Pharmacol. 2020. PMID: 32194409 Free PMC article. Review.
-
Tumor microenvironment and fibroblast activation protein inhibitor (FAPI) PET: developments toward brain imaging.Front Nucl Med. 2023 Jul 18;3:1183471. doi: 10.3389/fnume.2023.1183471. eCollection 2023. Front Nucl Med. 2023. PMID: 39355017 Free PMC article. Review.
References
-
- Patel S, Bhatnagar A, Wear C, Osiro S, Gabriel A, Kimball D, et al. Are pediatric brain tumors on the rise in the USA? Significant incidence and survival findings from the SEER database analysis. Child Nerv Syst. 2014;30:147–54. - PubMed
-
- Deorah S, Lynch CF, Sibenaller ZA, Ryken TC. Trends in brain cancer incidence and survival in the United States: Surveillance, Epidemiology, and End Results Program, 1973 to 2001. Neurosurgical focus. 2006;20:E1. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

