Respiratory Support for Very Low Birth Weight Infants Receiving Dexamethasone
- PMID: 28108103
- PMCID: PMC5368005
- DOI: 10.1016/j.jpeds.2016.12.035
Respiratory Support for Very Low Birth Weight Infants Receiving Dexamethasone
Abstract
Objective: To assess how neonatal intensive care units followed the American Academy of Pediatrics guidelines for use of dexamethasone in preterm infants by evaluating respiratory support at the time of dexamethasone administration.
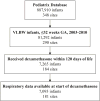
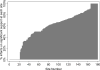
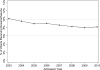
Study design: This is an observational study of infants discharged from one of 290 neonatal intensive care units from 2003 to 2010. The cohort included very low birth weight (<1500 g birth weight) infants born at ≤32 weeks gestational age. The main outcome was respiratory support at time of exposure to dexamethasone. Significant respiratory support was defined as invasive respiratory support (conventional or high-frequency ventilation) with a fraction of inspired oxygen (FiO2) > 0.3.
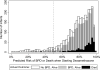
Results: Of 81 292 infants; 7093 (9%) received dexamethasone. At the time that dexamethasone was initiated, 4604 (65%) of infants were on significant respiratory support.
Conclusions: In accordance with the American Academy of Pediatrics recommendations, a majority of infants were on significant respiratory support when receiving dexamethasone, yet a substantial number of infants still received dexamethasone on less than significant respiratory support. Further research on reducing dexamethasone use in premature infants is required to decrease the risk of neurodevelopmental impairment.
Keywords: bronchopulmonary dysplasia; chronic lung disease; dexamethasone; very low birth weight.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Randomized, double-blinded trial of low-dose dexamethasone: II. Functional residual capacity and pulmonary outcome in very low birth weight infants at risk for bronchopulmonary dysplasia.Pediatr Pulmonol. 2004 Jul;38(1):55-63. doi: 10.1002/ppul.20037. Pediatr Pulmonol. 2004. PMID: 15170874 Clinical Trial.
-
A randomized trial of moderately early low-dose dexamethasone therapy in very low birth weight infants: dynamic pulmonary mechanics, oxygenation, and ventilation.Pediatrics. 2002 Feb;109(2):262-8. doi: 10.1542/peds.109.2.262. Pediatrics. 2002. PMID: 11826205 Clinical Trial.
-
First intention high-frequency oscillatory and conventional mechanical ventilation in premature infants without antenatal glucocorticoid prophylaxis.Pediatr Crit Care Med. 2012 Jan;13(1):72-9. doi: 10.1097/PCC.0b013e318219673e. Pediatr Crit Care Med. 2012. PMID: 21499177 Clinical Trial.
-
Inhalation or instillation of steroids for the prevention of bronchopulmonary dysplasia.Neonatology. 2015;107(4):358-9. doi: 10.1159/000381132. Epub 2015 Jun 5. Neonatology. 2015. PMID: 26044104 Review.
-
Early surfactant administration with brief ventilation vs selective surfactant and continued mechanical ventilation for preterm infants with or at risk for RDS.Cochrane Database Syst Rev. 2002;(2):CD003063. doi: 10.1002/14651858.CD003063. Cochrane Database Syst Rev. 2002. PMID: 12076469 Review.
Cited by
-
Absence of relationship between serum cortisol and critical illness in premature infants.Arch Dis Child Fetal Neonatal Ed. 2021 Jul;106(4):408-412. doi: 10.1136/archdischild-2020-319970. Epub 2021 Feb 4. Arch Dis Child Fetal Neonatal Ed. 2021. PMID: 33541918 Free PMC article.
-
Presumed adrenal insufficiency in neonates treated with corticosteroids for the prevention of bronchopulmonary dysplasia.J Perinatol. 2022 Jan;42(1):65-71. doi: 10.1038/s41372-021-01251-y. Epub 2021 Nov 1. J Perinatol. 2022. PMID: 34725449
-
Effect of Hydrocortisone Therapy Initiated 7 to 14 Days After Birth on Mortality or Bronchopulmonary Dysplasia Among Very Preterm Infants Receiving Mechanical Ventilation: A Randomized Clinical Trial.JAMA. 2019 Jan 29;321(4):354-363. doi: 10.1001/jama.2018.21443. JAMA. 2019. PMID: 30694322 Free PMC article. Clinical Trial.
References
-
- Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR, et al. Trends in neonatal morbidity and mortality for very low birthweight infants. American journal of obstetrics and gynecology. 2007 Feb;196(2):147 e1–8. - PubMed
-
- Van Marter LJ. Epidemiology of bronchopulmonary dysplasia. Semin Fetal Neonatal Med. 2009 Dec;14(6):358–66. - PubMed
-
- Halliday HL, Ehrenkranz RA. Early postnatal (<96 hours) corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database Syst Rev. 2001;(1):CD001146. - PubMed
-
- Yeh TF, Lin YJ, Huang CC, Chen YJ, Lin CH, Lin HC, et al. Early dexamethasone therapy in preterm infants: a follow-up study. Pediatrics. 1998 May;101(5):E7. - PubMed
-
- Yeh TF, Torre JA, Rastogi A, Anyebuno MA, Pildes RS. Early postnatal dexamethasone therapy in premature infants with severe respiratory distress syndrome: a double-blind, controlled study. The Journal of pediatrics. 1990 Aug;117(2 Pt 1):273–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

