6-Paradol and 6-Shogaol, the Pungent Compounds of Ginger, Promote Glucose Utilization in Adipocytes and Myotubes, and 6-Paradol Reduces Blood Glucose in High-Fat Diet-Fed Mice
- PMID: 28106738
- PMCID: PMC5297801
- DOI: 10.3390/ijms18010168
6-Paradol and 6-Shogaol, the Pungent Compounds of Ginger, Promote Glucose Utilization in Adipocytes and Myotubes, and 6-Paradol Reduces Blood Glucose in High-Fat Diet-Fed Mice
Abstract
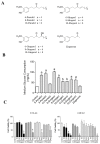
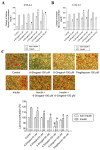
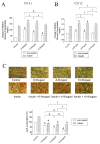
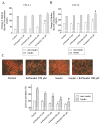
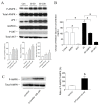
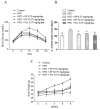
The anti-diabetic activity of ginger powder (Zingiber officinale) has been recently promoted, with the recommendation to be included as one of the dietary supplements for diabetic patients. However, previous studies presented different results, which may be caused by degradation and metabolic changes of ginger components, gingerols, shogaols and paradols. Therefore, we prepared 10 ginger active components, namely 6-, 8-, 10-paradols, 6-, 8-, 10-shogaols, 6-, 8-, 10-gingerols and zingerone, and evaluated their anti-hyperglycemic activity. Among the tested compounds, 6-paradol and 6-shogaol showed potent activity in stimulating glucose utilization by 3T3-L1 adipocytes and C2C12 myotubes. The effects were attributed to the increase in 5' adenosine monophosphate-activated protein kinase (AMPK) phosphorylation in 3T3-L1 adipocytes. 6-Paradol, the major metabolite of 6-shogaol, was utilized in an in vivo assay and significantly reduced blood glucose, cholesterol and body weight in high-fat diet-fed mice.
Keywords: 3T3-L1 adipocytes; 6-paradol; C2C12 myotubes; diabetes mellitus; high-fat diet-fed mice; shogaols.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Gingerols of Zingiber officinale enhance glucose uptake by increasing cell surface GLUT4 in cultured L6 myotubes.Planta Med. 2012 Sep;78(14):1549-55. doi: 10.1055/s-0032-1315041. Epub 2012 Jul 24. Planta Med. 2012. PMID: 22828920
-
Characterization of thiol-conjugated metabolites of ginger components shogaols in mouse and human urine and modulation of the glutathione levels in cancer cells by [6]-shogaol.Mol Nutr Food Res. 2013 Mar;57(3):447-58. doi: 10.1002/mnfr.201200679. Epub 2013 Jan 16. Mol Nutr Food Res. 2013. PMID: 23322393 Free PMC article.
-
Formation of 6-, 8- and 10-Shogaol in Ginger through Application of Different Drying Methods: Altered Antioxidant and Antimicrobial Activity.Molecules. 2018 Jul 5;23(7):1646. doi: 10.3390/molecules23071646. Molecules. 2018. PMID: 29976903 Free PMC article.
-
Cancer preventive properties of ginger: a brief review.Food Chem Toxicol. 2007 May;45(5):683-90. doi: 10.1016/j.fct.2006.11.002. Epub 2006 Nov 12. Food Chem Toxicol. 2007. PMID: 17175086 Review.
-
Update on the chemopreventive effects of ginger and its phytochemicals.Crit Rev Food Sci Nutr. 2011 Jul;51(6):499-523. doi: 10.1080/10408391003698669. Crit Rev Food Sci Nutr. 2011. PMID: 21929329 Review.
Cited by
-
The Effect of Therapeutic Doses of Culinary Spices in Metabolic Syndrome: A Randomized Controlled Trial.Nutrients. 2024 May 29;16(11):1685. doi: 10.3390/nu16111685. Nutrients. 2024. PMID: 38892617 Free PMC article. Clinical Trial.
-
Association of dietary inflammatory index with constipation: Evidence from the National Health and Nutrition Examination Survey.Food Sci Nutr. 2024 Jan 17;12(3):2122-2130. doi: 10.1002/fsn3.3914. eCollection 2024 Mar. Food Sci Nutr. 2024. PMID: 38455207 Free PMC article.
-
In vitro and in silico cholinesterase inhibitory potential of metabolites from Laurencia snackeyi (Weber-van Bosse) M. Masuda.3 Biotech. 2023 Oct;13(10):337. doi: 10.1007/s13205-023-03725-6. Epub 2023 Sep 10. 3 Biotech. 2023. PMID: 37701628 Free PMC article.
-
Plant-Derived Nutraceuticals Involved in Body Weight Control by Modulating Gene Expression.Plants (Basel). 2023 Jun 11;12(12):2273. doi: 10.3390/plants12122273. Plants (Basel). 2023. PMID: 37375898 Free PMC article. Review.
-
Modulatory Effect of Medicinal Plants and Their Active Constituents on ATP-Sensitive Potassium Channels (KATP) in Diabetes.Pharmaceuticals (Basel). 2023 Mar 31;16(4):523. doi: 10.3390/ph16040523. Pharmaceuticals (Basel). 2023. PMID: 37111281 Free PMC article. Review.
References
-
- White B. Ginger: An overview. Am. Fam. Physician. 2007;75:1689–1691. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

