A Systems Approach Reveals MAVS Signaling in Myeloid Cells as Critical for Resistance to Ebola Virus in Murine Models of Infection
- PMID: 28099857
- PMCID: PMC5289750
- DOI: 10.1016/j.celrep.2016.12.069
A Systems Approach Reveals MAVS Signaling in Myeloid Cells as Critical for Resistance to Ebola Virus in Murine Models of Infection
Abstract
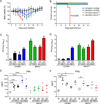
The unprecedented 2013-2016 outbreak of Ebola virus (EBOV) resulted in over 11,300 human deaths. Host resistance to RNA viruses requires RIG-I-like receptor (RLR) signaling through the adaptor protein, mitochondrial antiviral signaling protein (MAVS), but the role of RLR-MAVS in orchestrating anti-EBOV responses in vivo is not known. Here we apply a systems approach to MAVS-/- mice infected with either wild-type or mouse-adapted EBOV. MAVS controlled EBOV replication through the expression of IFNα, regulation of inflammatory responses in the spleen, and prevention of cell death in the liver, with macrophages implicated as a major cell type influencing host resistance. A dominant role for RLR signaling in macrophages was confirmed following conditional MAVS deletion in LysM+ myeloid cells. These findings reveal tissue-specific MAVS-dependent transcriptional pathways associated with resistance to EBOV, and they demonstrate that EBOV adaptation to cause disease in mice involves changes in two distinct events, RLR-MAVS antagonism and suppression of RLR-independent IFN-I responses.
Keywords: Ebola virus; MAVS; RLR; conditional; interferon; knockout; macrophages; mouse adapted.
Published by Elsevier Inc.
Figures



Similar articles
-
Ebola Virus Replication and Disease Without Immunopathology in Mice Expressing Transgenes to Support Human Myeloid and Lymphoid Cell Engraftment.J Infect Dis. 2016 Oct 15;214(suppl 3):S308-S318. doi: 10.1093/infdis/jiw248. Epub 2016 Sep 6. J Infect Dis. 2016. PMID: 27601621 Free PMC article.
-
Lactate Is a Natural Suppressor of RLR Signaling by Targeting MAVS.Cell. 2019 Jun 27;178(1):176-189.e15. doi: 10.1016/j.cell.2019.05.003. Epub 2019 May 30. Cell. 2019. PMID: 31155231 Free PMC article.
-
Oropouche virus infection and pathogenesis are restricted by MAVS, IRF-3, IRF-7, and type I interferon signaling pathways in nonmyeloid cells.J Virol. 2015 May;89(9):4720-37. doi: 10.1128/JVI.00077-15. Epub 2015 Feb 25. J Virol. 2015. PMID: 25717109 Free PMC article.
-
Development of a murine model for aerosolized ebolavirus infection using a panel of recombinant inbred mice.Viruses. 2012 Dec 3;4(12):3468-93. doi: 10.3390/v4123468. Viruses. 2012. PMID: 23207275 Free PMC article. Review.
-
Clinical features and pathobiology of Ebolavirus infection.J Autoimmun. 2014 Dec;55:1-9. doi: 10.1016/j.jaut.2014.09.001. Epub 2014 Sep 26. J Autoimmun. 2014. PMID: 25260583 Review.
Cited by
-
Picking up a Fight: Fine Tuning Mitochondrial Innate Immune Defenses Against RNA Viruses.Front Microbiol. 2020 Aug 31;11:1990. doi: 10.3389/fmicb.2020.01990. eCollection 2020. Front Microbiol. 2020. PMID: 32983015 Free PMC article. Review.
-
Ebola Virus Uses Tunneling Nanotubes as an Alternate Route of Dissemination.J Infect Dis. 2023 Nov 13;228(Suppl 7):S522-S535. doi: 10.1093/infdis/jiad400. J Infect Dis. 2023. PMID: 37723997 Free PMC article.
-
Coordinated regulation of interferon and inflammasome signaling pathways by SARS-CoV-2 proteins.J Microbiol. 2022 Mar;60(3):300-307. doi: 10.1007/s12275-022-1502-8. Epub 2022 Jan 28. J Microbiol. 2022. PMID: 35089584 Free PMC article. Review.
-
Macrophage infection, activation, and histopathological findings in ebolavirus infection.Front Cell Infect Microbiol. 2022 Oct 12;12:1023557. doi: 10.3389/fcimb.2022.1023557. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36310868 Free PMC article. Review.
-
Role of Type I Interferons on Filovirus Pathogenesis.Vaccines (Basel). 2019 Feb 20;7(1):22. doi: 10.3390/vaccines7010022. Vaccines (Basel). 2019. PMID: 30791589 Free PMC article. Review.
References
-
- Albarino CG, Wiggleton Guerrero L, Lo MK, Nichol ST, Towner JS. Development of a reverse genetics system to generate a recombinant Ebola virus Makona expressing a green fluorescent protein. Virology. 2015;484:259–264. - PubMed
-
- Bradfute SB, Braun DR, Shamblin JD, Geisbert JB, Paragas J, Garrison A, Hensley LE, Geisbert TW. Lymphocyte death in a mouse model of Ebola virus infection. J. Infect. Dis. 2007;196(Suppl 2):S296–S304. - PubMed
-
- Bray M. The role of the Type I interferon response in the resistance of mice to filovirus infection. J. Gen. Virol. 2001;82:1365–1373. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

