Identification of a Continuous Neutralizing Epitope within UL128 of Human Cytomegalovirus
- PMID: 28077639
- PMCID: PMC5331811
- DOI: 10.1128/JVI.01857-16
Identification of a Continuous Neutralizing Epitope within UL128 of Human Cytomegalovirus
Abstract
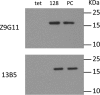
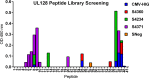
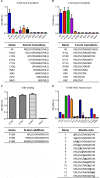
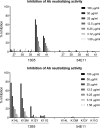
As human cytomegalovirus (HCMV) is the most common infectious cause of fetal anomalies during pregnancy, development of a vaccine that prevents HCMV infection is considered a global health priority. Although HCMV immune correlates of protection are only poorly defined, neutralizing antibodies (NAb) targeting the envelope pentamer complex (PC) composed of the subunits gH, gL, UL128, UL130, and UL131A are thought to contribute to the prevention of HCMV infection. Here, we describe a continuous target sequence within UL128 that is recognized by a previously isolated potent PC-specific NAb termed 13B5. By using peptide-based scanning procedures, we identified a 13-amino-acid-long target sequence at the UL128 C terminus that binds the 13B5 antibody with an affinity similar to that of the purified PC. In addition, the 13B5 binding site is universally conserved in HCMV, contains a previously described UL128/gL interaction site, and interferes with the 13B5 neutralizing function, indicating that the 13B5 epitope sequence is located within the PC at a site of critical importance for HCMV neutralization. Vaccination of mice with peptides containing the 13B5 target sequence resulted in the robust stimulation of binding antibodies and, in a subset of immunized animals, in the induction of detectable NAb, supporting that the identified 13B5 target sequence constitutes a PC-specific neutralizing epitope. These findings provide evidence for the discovery of a continuous neutralizing epitope within the UL128 subunit of the PC that could be an important target of humoral immune responses that are involved in protection against congenital HCMV infection.IMPORTANCE Neutralizing antibodies (NAb) targeting the human cytomegalovirus (HCMV) envelope pentamer complex (PC) are thought to be important for preventing HCMV transmission from the mother to the fetus, thereby mitigating severe developmental disabilities in newborns. However, the epitope sequences within the PC that are recognized by these potentially protective antibody responses are only poorly defined. Here, we provide evidence for the existence of a highly conserved, continuous, PC-specific epitope sequence that appears to be located within the PC at a subunit interaction site of critical importance for HCMV neutralization. These discoveries provide insights into a continuous PC-specific neutralizing epitope, which could be an important target for a vaccine formulation to interfere with congenital HCMV infection.
Keywords: HCMV; UL128; epitope; pentamer complex; peptide.
Copyright © 2017 American Society for Microbiology.
Figures

Similar articles
-
Vaccine-Derived Neutralizing Antibodies to the Human Cytomegalovirus gH/gL Pentamer Potently Block Primary Cytotrophoblast Infection.J Virol. 2015 Dec;89(23):11884-98. doi: 10.1128/JVI.01701-15. Epub 2015 Sep 16. J Virol. 2015. PMID: 26378171 Free PMC article.
-
Human cytomegalovirus vaccine based on the envelope gH/gL pentamer complex.PLoS Pathog. 2014 Nov 20;10(11):e1004524. doi: 10.1371/journal.ppat.1004524. eCollection 2014 Nov. PLoS Pathog. 2014. PMID: 25412505 Free PMC article.
-
Dense Bodies of a gH/gL/UL128/UL130/UL131 Pentamer-Repaired Towne Strain of Human Cytomegalovirus Induce an Enhanced Neutralizing Antibody Response.J Virol. 2019 Aug 13;93(17):e00931-19. doi: 10.1128/JVI.00931-19. Print 2019 Sep 1. J Virol. 2019. PMID: 31189713 Free PMC article.
-
Neutralization of Human Cytomegalovirus Entry into Fibroblasts and Epithelial Cells.Vaccines (Basel). 2017 Oct 31;5(4):39. doi: 10.3390/vaccines5040039. Vaccines (Basel). 2017. PMID: 29088098 Free PMC article. Review.
-
Role of human cytomegalovirus (HCMV)-specific antibody in HCMV-infected pregnant women.Early Hum Dev. 2014 Mar;90 Suppl 1:S32-4. doi: 10.1016/S0378-3782(14)70011-8. Early Hum Dev. 2014. PMID: 24709453 Review.
Cited by
-
Targeting Human-Cytomegalovirus-Infected Cells by Redirecting T Cells Using an Anti-CD3/Anti-Glycoprotein B Bispecific Antibody.Antimicrob Agents Chemother. 2017 Dec 21;62(1):e01719-17. doi: 10.1128/AAC.01719-17. Print 2018 Jan. Antimicrob Agents Chemother. 2017. PMID: 29038280 Free PMC article.
-
Human Cytomegalovirus Particles Treated with Specific Antibodies Induce Intrinsic and Adaptive but Not Innate Immune Responses.J Virol. 2017 Oct 27;91(22):e00678-17. doi: 10.1128/JVI.00678-17. Print 2017 Nov 15. J Virol. 2017. PMID: 28878085 Free PMC article.
-
HCMV Envelope Glycoprotein Diversity Demystified.Front Microbiol. 2019 May 15;10:1005. doi: 10.3389/fmicb.2019.01005. eCollection 2019. Front Microbiol. 2019. PMID: 31156572 Free PMC article.
-
Exploiting 2A peptides to elicit potent neutralizing antibodies by a multi-subunit herpesvirus glycoprotein complex.J Virol Methods. 2018 Jan;251:30-37. doi: 10.1016/j.jviromet.2017.10.006. Epub 2017 Oct 6. J Virol Methods. 2018. PMID: 28989096 Free PMC article.
-
Chemokines encoded by herpesviruses.J Leukoc Biol. 2017 Nov;102(5):1199-1217. doi: 10.1189/jlb.4RU0417-145RR. Epub 2017 Aug 28. J Leukoc Biol. 2017. PMID: 28848041 Free PMC article. Review.
References
-
- Britt W. 2008. Manifestations of human cytomegalovirus infection: proposed mechanisms of acute and chronic disease. Curr Top Microbiol Immunol 325:417–470. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

