Fibroblast drug scavenging increases intratumoural gemcitabine accumulation in murine pancreas cancer
- PMID: 28077438
- PMCID: PMC5868285
- DOI: 10.1136/gutjnl-2016-311954
Fibroblast drug scavenging increases intratumoural gemcitabine accumulation in murine pancreas cancer
Abstract
Objective: Desmoplasia and hypovascularity are thought to impede drug delivery in pancreatic ductal adenocarcinoma (PDAC). However, stromal depletion approaches have failed to show clinical responses in patients. Here, we aimed to revisit the role of the tumour microenvironment as a physical barrier for gemcitabine delivery.
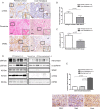
Design: Gemcitabine metabolites were analysed in LSL-KrasG12D/+ ; LSL-Trp53R172H/+ ; Pdx-1-Cre (KPC) murine tumours and matched liver metastases, primary tumour cell lines, cancer-associated fibroblasts (CAFs) and pancreatic stellate cells (PSCs) by liquid chromatography-mass spectrometry/mass spectrometry. Functional and preclinical experiments, as well as expression analysis of stromal markers and gemcitabine metabolism pathways were performed in murine and human specimen to investigate the preclinical implications and the mechanism of gemcitabine accumulation.
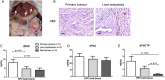
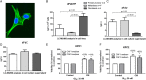
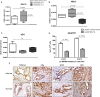
Results: Gemcitabine accumulation was significantly enhanced in fibroblast-rich tumours compared with liver metastases and normal liver. In vitro, significantly increased concentrations of activated 2',2'-difluorodeoxycytidine-5'-triphosphate (dFdCTP) and greatly reduced amounts of the inactive gemcitabine metabolite 2',2'-difluorodeoxyuridine were detected in PSCs and CAFs. Mechanistically, key metabolic enzymes involved in gemcitabine inactivation such as hydrolytic cytosolic 5'-nucleotidases (Nt5c1A, Nt5c3) were expressed at low levels in CAFs in vitro and in vivo, and recombinant expression of Nt5c1A resulted in decreased intracellular dFdCTP concentrations in vitro. Moreover, gemcitabine treatment in KPC mice reduced the number of liver metastases by >50%.
Conclusions: Our findings suggest that fibroblast drug scavenging may contribute to the clinical failure of gemcitabine in desmoplastic PDAC. Metabolic targeting of CAFs may thus be a promising strategy to enhance the antiproliferative effects of gemcitabine.
Keywords: CHEMOTHERAPY; DRUG METABOLISM; PANCREATIC CANCER; PANCREATIC FIBROSIS.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
Competing interests: None declared.
Figures

Similar articles
-
Cytosolic 5'-nucleotidase 1A is overexpressed in pancreatic cancer and mediates gemcitabine resistance by reducing intracellular gemcitabine metabolites.EBioMedicine. 2019 Feb;40:394-405. doi: 10.1016/j.ebiom.2019.01.037. Epub 2019 Jan 30. EBioMedicine. 2019. PMID: 30709769 Free PMC article.
-
Expression of gemcitabine metabolizing enzymes and stromal components reveal complexities of preclinical pancreatic cancer models for therapeutic testing.Neoplasia. 2024 Jul;53:101002. doi: 10.1016/j.neo.2024.101002. Epub 2024 May 13. Neoplasia. 2024. PMID: 38744194 Free PMC article.
-
Cadherin 11 Promotes Immunosuppression and Extracellular Matrix Deposition to Support Growth of Pancreatic Tumors and Resistance to Gemcitabine in Mice.Gastroenterology. 2021 Mar;160(4):1359-1372.e13. doi: 10.1053/j.gastro.2020.11.044. Epub 2020 Dec 9. Gastroenterology. 2021. PMID: 33307028 Free PMC article.
-
Tumour-stroma interactions in pancreatic ductal adenocarcinoma: rationale and current evidence for new therapeutic strategies.Cancer Treat Rev. 2014 Feb;40(1):118-28. doi: 10.1016/j.ctrv.2013.04.004. Epub 2013 Jul 9. Cancer Treat Rev. 2014. PMID: 23849556 Review.
-
Harnessing metabolic dependencies in pancreatic cancers.Nat Rev Gastroenterol Hepatol. 2021 Jul;18(7):482-492. doi: 10.1038/s41575-021-00431-7. Epub 2021 Mar 19. Nat Rev Gastroenterol Hepatol. 2021. PMID: 33742165 Free PMC article. Review.
Cited by
-
KRAS, A Prime Mediator in Pancreatic Lipid Synthesis through Extra Mitochondrial Glutamine and Citrate Metabolism.Int J Mol Sci. 2021 May 11;22(10):5070. doi: 10.3390/ijms22105070. Int J Mol Sci. 2021. PMID: 34064761 Free PMC article. Review.
-
Epigenetic Therapeutic Strategies to Target Molecular and Cellular Heterogeneity in Pancreatic Cancer.Visc Med. 2022 Feb;38(1):11-19. doi: 10.1159/000519859. Epub 2021 Nov 19. Visc Med. 2022. PMID: 35291698 Free PMC article. Review.
-
Preclinical Evaluation of 1,2-Diamino-4,5-Dibromobenzene in Genetically Engineered Mouse Models of Pancreatic Cancer.Cells. 2019 Jun 9;8(6):563. doi: 10.3390/cells8060563. Cells. 2019. PMID: 31181844 Free PMC article.
-
Organoid Profiling Identifies Common Responders to Chemotherapy in Pancreatic Cancer.Cancer Discov. 2018 Sep;8(9):1112-1129. doi: 10.1158/2159-8290.CD-18-0349. Epub 2018 May 31. Cancer Discov. 2018. PMID: 29853643 Free PMC article.
-
Low expression of SPARC in gastric cancer-associated fibroblasts leads to stemness transformation and 5-fluorouracil resistance in gastric cancer.Cancer Cell Int. 2019 May 21;19:137. doi: 10.1186/s12935-019-0844-8. eCollection 2019. Cancer Cell Int. 2019. PMID: 31139014 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
