Hepatocyte TAZ/WWTR1 Promotes Inflammation and Fibrosis in Nonalcoholic Steatohepatitis
- PMID: 28068223
- PMCID: PMC5226184
- DOI: 10.1016/j.cmet.2016.09.016
Hepatocyte TAZ/WWTR1 Promotes Inflammation and Fibrosis in Nonalcoholic Steatohepatitis
Abstract
Nonalcoholic steatohepatitis (NASH) is a leading cause of liver disease worldwide. However, the molecular basis of how benign steatosis progresses to NASH is incompletely understood, which has limited the identification of therapeutic targets. Here we show that the transcription regulator TAZ (WWTR1) is markedly higher in hepatocytes in human and murine NASH liver than in normal or steatotic liver. Most importantly, silencing of hepatocyte TAZ in murine models of NASH prevented or reversed hepatic inflammation, hepatocyte death, and fibrosis, but not steatosis. Moreover, hepatocyte-targeted expression of TAZ in a model of steatosis promoted NASH features, including fibrosis. In vitro and in vivo mechanistic studies revealed that a key mechanism linking hepatocyte TAZ to NASH fibrosis is TAZ/TEA domain (TEAD)-mediated induction of Indian hedgehog (Ihh), a secretory factor that activates fibrogenic genes in hepatic stellate cells. In summary, TAZ represents a previously unrecognized factor that contributes to the critical process of steatosis-to-NASH progression.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
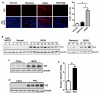
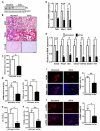
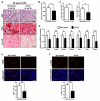
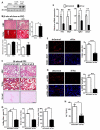

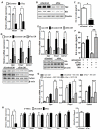
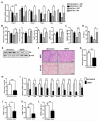
Comment in
-
NASH: Understanding how steatosis progresses to NASH.Nat Rev Endocrinol. 2017 Jan;13(1):5. doi: 10.1038/nrendo.2016.187. Epub 2016 Nov 11. Nat Rev Endocrinol. 2017. PMID: 27834389 No abstract available.
-
Devilish Effects of Taz in Nonalcoholic Steatohepatitis.Cell Metab. 2016 Dec 13;24(6):771-772. doi: 10.1016/j.cmet.2016.10.006. Epub 2016 Oct 27. Cell Metab. 2016. PMID: 28068221
-
Liver cholesterol matters.Aging (Albany NY). 2020 Oct 26;12(20):19828-19829. doi: 10.18632/aging.104208. Epub 2020 Oct 26. Aging (Albany NY). 2020. PMID: 33125343 Free PMC article. No abstract available.
Similar articles
-
Cholesterol Stabilizes TAZ in Hepatocytes to Promote Experimental Non-alcoholic Steatohepatitis.Cell Metab. 2020 May 5;31(5):969-986.e7. doi: 10.1016/j.cmet.2020.03.010. Epub 2020 Apr 6. Cell Metab. 2020. PMID: 32259482 Free PMC article.
-
TAZ-induced Cybb contributes to liver tumor formation in non-alcoholic steatohepatitis.J Hepatol. 2022 Apr;76(4):910-920. doi: 10.1016/j.jhep.2021.11.031. Epub 2021 Dec 11. J Hepatol. 2022. PMID: 34902531 Free PMC article.
-
Hepatocyte nuclear receptor SHP suppresses inflammation and fibrosis in a mouse model of nonalcoholic steatohepatitis.J Biol Chem. 2018 Jun 1;293(22):8656-8671. doi: 10.1074/jbc.RA117.001653. Epub 2018 Apr 17. J Biol Chem. 2018. PMID: 29666185 Free PMC article.
-
Mechanisms of Fibrosis Development in Nonalcoholic Steatohepatitis.Gastroenterology. 2020 May;158(7):1913-1928. doi: 10.1053/j.gastro.2019.11.311. Epub 2020 Feb 8. Gastroenterology. 2020. PMID: 32044315 Free PMC article. Review.
-
Maladaptive regeneration - the reawakening of developmental pathways in NASH and fibrosis.Nat Rev Gastroenterol Hepatol. 2021 Feb;18(2):131-142. doi: 10.1038/s41575-020-00365-6. Epub 2020 Oct 13. Nat Rev Gastroenterol Hepatol. 2021. PMID: 33051603 Free PMC article. Review.
Cited by
-
Emerging novel targets for nonalcoholic fatty liver disease treatment: Evidence from recent basic studies.World J Gastroenterol. 2023 Jan 7;29(1):75-95. doi: 10.3748/wjg.v29.i1.75. World J Gastroenterol. 2023. PMID: 36683713 Free PMC article. Review.
-
A Novel 2-Hit Zebrafish Model to Study Early Pathogenesis of Non-Alcoholic Fatty Liver Disease.Biomedicines. 2022 Feb 17;10(2):479. doi: 10.3390/biomedicines10020479. Biomedicines. 2022. PMID: 35203687 Free PMC article.
-
A systems biology approach to study non-alcoholic fatty liver (NAFL) in women with obesity.iScience. 2022 Aug 5;25(8):104828. doi: 10.1016/j.isci.2022.104828. eCollection 2022 Aug 19. iScience. 2022. PMID: 35992074 Free PMC article.
-
Relation Between Immunohistochemical Expression of Hippo Pathway Effectors and Chronic Hepatitis Induced Fibrosis in Egyptian Patients.Turk Patoloji Derg. 2020;36(1):48-63. doi: 10.5146/tjpath.2019.01463. Turk Patoloji Derg. 2020. PMID: 31282549 Free PMC article.
-
Nanotechnology in Drug Delivery for Liver Fibrosis.Front Mol Biosci. 2022 Jan 11;8:804396. doi: 10.3389/fmolb.2021.804396. eCollection 2021. Front Mol Biosci. 2022. PMID: 35087870 Free PMC article. Review.
References
-
- Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Keach JC, Lafferty HD, Stahler A, et al. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015a;149:389–397. e310. - PMC - PubMed
-
- Angulo P, Machado MV, Diehl AM. Fibrosis in nonalcoholic Fatty liver disease: mechanisms and clinical implications. Semin Liver Dis. 2015b;35:132–145. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

