Surface engineering tumor cells with adjuvant-loaded particles for use as cancer vaccines
- PMID: 28057523
- PMCID: PMC5309920
- DOI: 10.1016/j.jconrel.2016.12.036
Surface engineering tumor cells with adjuvant-loaded particles for use as cancer vaccines
Abstract
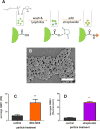
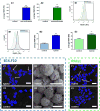
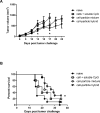
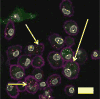
Cell surface engineering is an expanding field and whilst extensive research has been performed decorating cell surfaces with biomolecules, the engineering of cell surfaces with particles has been a largely unexploited area. This study reports on the assembly of cell-particle hybrids where irradiated tumor cells were surface engineered with adjuvant-loaded, biodegradable, biocompatible, polymeric particles, with the aim of generating a construct capable of functioning as a therapeutic cancer vaccine. Successfully assembled cell-particle hybrids presented here comprised either melanoma cells or prostate cancer cells stably adorned with Toll-like receptor-9 ligand-loaded particles using streptavidin-biotin cross-linking. Both cell-particle assemblies were tested in vivo for their potential as therapeutic cancer vaccines yielding promising therapeutic results for the prostate cancer model. The ramifications of results obtained for both tumor models are openly discussed.
Keywords: Cancer vaccines; Cell surface engineering; PLGA particles; Streptavidin-biotin cross-linking.
Copyright © 2017 Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Diaminosulfide based polymer microparticles as cancer vaccine delivery systems.J Control Release. 2015 Dec 28;220(Pt B):682-90. doi: 10.1016/j.jconrel.2015.09.002. Epub 2015 Sep 8. J Control Release. 2015. PMID: 26359124 Free PMC article.
-
Development and Evaluation of Biodegradable Particles Coloaded With Antigen and the Toll-Like Receptor Agonist, Pentaerythritol Lipid A, as a Cancer Vaccine.J Pharm Sci. 2016 Mar;105(3):1173-9. doi: 10.1016/j.xphs.2015.11.042. Epub 2016 Jan 30. J Pharm Sci. 2016. PMID: 26886334 Free PMC article.
-
Biotinylated Streptavidin Surface Coating Improves the Efficacy of a PLGA Microparticle-Based Cancer Vaccine.Bioconjug Chem. 2020 Sep 16;31(9):2147-2157. doi: 10.1021/acs.bioconjchem.0c00347. Epub 2020 Aug 31. Bioconjug Chem. 2020. PMID: 32786363
-
Targeting dendritic cells with nano-particulate PLGA cancer vaccine formulations.Adv Drug Deliv Rev. 2011 Sep 10;63(10-11):943-55. doi: 10.1016/j.addr.2011.05.021. Epub 2011 Jun 6. Adv Drug Deliv Rev. 2011. PMID: 21679733 Review.
-
Particulate Systems Based on Poly(Lactic-co-Glycolic)Acid (pLGA) for Immunotherapy of Cancer.Curr Pharm Des. 2015;21(29):4201-16. doi: 10.2174/1381612821666150901100247. Curr Pharm Des. 2015. PMID: 26323429 Review.
Cited by
-
Advances in Immunotherapy for Melanoma: A Comprehensive Review.Mediators Inflamm. 2017;2017:3264217. doi: 10.1155/2017/3264217. Epub 2017 Aug 1. Mediators Inflamm. 2017. PMID: 28848246 Free PMC article. Review.
-
Functionalized Peptide-Based Nanoparticles for Targeted Cancer Nanotherapeutics: A State-of-the-Art Review.ACS Omega. 2022 Oct 5;7(41):36092-36107. doi: 10.1021/acsomega.2c03974. eCollection 2022 Oct 18. ACS Omega. 2022. PMID: 36278104 Free PMC article. Review.
-
The construction of a lymphoma cell-based, DC-targeted vaccine, and its application in lymphoma prevention and cure.Bioact Mater. 2020 Sep 22;6(3):697-711. doi: 10.1016/j.bioactmat.2020.09.002. eCollection 2021 Mar. Bioact Mater. 2020. PMID: 33005832 Free PMC article.
-
Toll-like receptor-targeted anti-tumor therapies: Advances and challenges.Front Immunol. 2022 Nov 21;13:1049340. doi: 10.3389/fimmu.2022.1049340. eCollection 2022. Front Immunol. 2022. PMID: 36479129 Free PMC article. Review.
-
Cell-Based Drug Delivery Systems with Innate Homing Capability as a Novel Nanocarrier Platform.Int J Nanomedicine. 2023 Jan 29;18:509-525. doi: 10.2147/IJN.S394389. eCollection 2023. Int J Nanomedicine. 2023. PMID: 36742991 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

