Electroacupuncture treatment partly promotes the recovery time of postoperative ileus by activating the vagus nerve but not regulating local inflammation
- PMID: 28051128
- PMCID: PMC5209726
- DOI: 10.1038/srep39801
Electroacupuncture treatment partly promotes the recovery time of postoperative ileus by activating the vagus nerve but not regulating local inflammation
Abstract
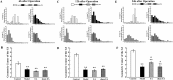
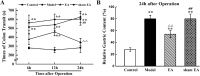
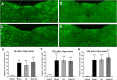
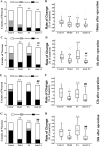
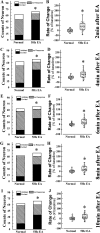
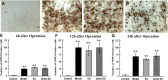
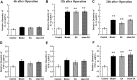
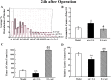
Postoperative ileus (POI) after abdominal surgery significantly lowers the life quality of patients and increase hospital costs. However, few treatment strategies have successfully shortened the duration of POI. Electroacupuncture (EA) is a modern way of administering acupuncture and widely used in various gastrointestinal (GI) diseases in the world. Here, we studied the effect of EA on POI and its underlying mechanisms. Intestinal manipulation resulted in significant delays of GI transit, colonic transit and gastric emptying. Surgery also up-regulated c-fos in nucleus of the solitary tract (NTS) and induced inflammation response in the small intestine. Further, operation and inhale anesthesia inhibited NTS neuron excitation duration for the whole observation time. EA administered at ST36 indeed shortened the recovery time of GI and colonic transit, and significantly increased the gastric emptying. EA also significantly activated the NTS neurons after operation. However, there was no anti-inflammation effect of EA during the whole experiment. Finally, atropine blocked the regulatory effect of EA on GI function, when it was injected after surgery, but not before surgery. Thus, the regulatory effect of EA on POI was mainly mediated by exciting NTS neurons to improve the GI tract transit function but not by activating cholinergic anti-inflammatory pathway.
Figures
Similar articles
-
Electroacupuncture treatment improves postoperative ileus by inhibiting the Th1 cell-mediated inflammatory response through the vagus nerve.Acupunct Med. 2024 Jun;42(3):123-132. doi: 10.1177/09645284241248466. Epub 2024 May 30. Acupunct Med. 2024. PMID: 38813841
-
Electroacupuncture alleviates intestinal inflammation via a distinct neuro-immune signal pathway in the treatment of postoperative ileus.Biomed Pharmacother. 2024 Apr;173:116387. doi: 10.1016/j.biopha.2024.116387. Epub 2024 Mar 11. Biomed Pharmacother. 2024. PMID: 38471276
-
Inhibition of sympathetic pathways restores postoperative ileus in the upper and lower gastrointestinal tract.J Gastroenterol Hepatol. 2007 Aug;22(8):1293-9. doi: 10.1111/j.1440-1746.2007.04915.x. J Gastroenterol Hepatol. 2007. PMID: 17688668
-
Emerging drugs for postoperative ileus.Expert Opin Emerg Drugs. 2007 Nov;12(4):619-26. doi: 10.1517/14728214.12.4.619. Expert Opin Emerg Drugs. 2007. PMID: 17979603 Review.
-
Electrical vagus nerve stimulation as a prophylaxis for SIRS and postoperative ileus.Auton Neurosci. 2021 Nov;235:102857. doi: 10.1016/j.autneu.2021.102857. Epub 2021 Jul 29. Auton Neurosci. 2021. PMID: 34343825 Review.
Cited by
-
Prokinetic effects of spinal cord stimulation and its autonomic mechanisms in dogs.Neurogastroenterol Motil. 2019 Jul;31(7):e13596. doi: 10.1111/nmo.13596. Epub 2019 Apr 14. Neurogastroenterol Motil. 2019. PMID: 30983068 Free PMC article.
-
Acupuncture for enhancing early recovery of bowel function in cancer: Protocol for a systematic review.Medicine (Baltimore). 2017 Apr;96(17):e6644. doi: 10.1097/MD.0000000000006644. Medicine (Baltimore). 2017. PMID: 28445263 Free PMC article.
-
Effectiveness and safety of acupuncture for postoperative ileus following gastrointestinal surgery: A systematic review and meta-analysis.PLoS One. 2022 Jul 18;17(7):e0271580. doi: 10.1371/journal.pone.0271580. eCollection 2022. PLoS One. 2022. PMID: 35849611 Free PMC article.
-
State-of-the-art colorectal disease: postoperative ileus.Int J Colorectal Dis. 2021 Sep;36(9):2017-2025. doi: 10.1007/s00384-021-03939-1. Epub 2021 May 11. Int J Colorectal Dis. 2021. PMID: 33977334 Free PMC article. Review.
-
Restoring brain health: Electroacupuncture at GB20 and LR3 for migraine mitigation through mitochondrial restoration.Brain Circ. 2024 Jun 26;10(2):154-161. doi: 10.4103/bc.bc_95_23. eCollection 2024 Apr-Jun. Brain Circ. 2024. PMID: 39036293 Free PMC article.
References
-
- Kalff J. C., Schraut W. H., Billiar T. R., Simmons R. L. & Bauer A. J. Role of inducible nitric oxide synthase in postoperative intestinal smooth muscle dysfunction in rodents. Gastroenterology 118, 316–327 (2000). - PubMed
-
- de Jonge W. J. et al.. Postoperative ileus is maintained by intestinal immune infiltrates that activate inhibitory neural pathways in mice. Gastroenterology 125, 1137–1147 (2003). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

