Investigation of a Novel Hepatitis B Virus Surface Antigen (HBsAg) Escape Mutant Affecting Immunogenicity
- PMID: 28045894
- PMCID: PMC5207502
- DOI: 10.1371/journal.pone.0167871
Investigation of a Novel Hepatitis B Virus Surface Antigen (HBsAg) Escape Mutant Affecting Immunogenicity
Abstract
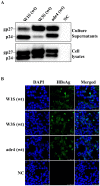
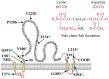
Mutation in the hepatitis B virus surface antigen (HBsAg) may affect the efficiency of diagnostic immunoassays or success of vaccinations using HBsAg. Thus, antigenicity and immunogenicity analyses of the mutated HBsAg are necessary to develop novel diagnostic tools and efficient vaccinations. Here, the in vitro antigenicity of three wild-type HBsAg open reading frames (ORFs) (adr4, W1S [subtype adr] and W3S [subtype adr]) isolated from clinically infected patients and nineteen synthesized single/double/multiple amino acid-substituted mutants were tested with commercial ELISA kits. Immunofluorescence staining of transfected cells and Western blot analysis confirmed that these ORFs were expressed at comparable levels in HEK-293 cells. W1S and adr4 were clearly detected, whereas W3S could not be detected. Using the same commercial immunoassay kit, we found that the single mutants, K120P and D123T, were marginally reactive, whereas W3S-aW1S and the double mutant, K120P/D123T, exhibited antigenicity roughly equivalent to the wild-type wako1S. On the other hand, the single mutants of W1S, P120K and T123D, significantly impaired the reactivity, while W1S-aW3S and the double mutant of W1S, P120K/T123D, resulted in a complete loss of antigenicity. In addition, ELISA revealed reduced HBs antigenicity of two mutants, W1S N146G and W1S Q129R/G145R. These commercial ELISA-based antigenic reactivities of HBsAg were also strongly correlated with the predicted Ai alterations of affected amino acids due to the specific mutation. In conclusion, this study showed for the first time that lysine (K120) and aspartate (D123) simultaneously affected HBsAg antigenicity, leading to diagnostic failure. These findings will improve diagnostic assays and vaccine development.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Biological significance of amino acid substitutions in hepatitis B surface antigen (HBsAg) for glycosylation, secretion, antigenicity and immunogenicity of HBsAg and hepatitis B virus replication.J Gen Virol. 2010 Feb;91(Pt 2):483-92. doi: 10.1099/vir.0.012740-0. Epub 2009 Oct 7. J Gen Virol. 2010. PMID: 19812261
-
PreS1 Mutations Alter the Large HBsAg Antigenicity of a Hepatitis B Virus Strain Isolated in Bangladesh.Int J Mol Sci. 2020 Jan 15;21(2):546. doi: 10.3390/ijms21020546. Int J Mol Sci. 2020. PMID: 31952213 Free PMC article.
-
The amino Acid residues at positions 120 to 123 are crucial for the antigenicity of hepatitis B surface antigen.J Clin Microbiol. 2007 Sep;45(9):2971-8. doi: 10.1128/JCM.00508-07. Epub 2007 Jul 3. J Clin Microbiol. 2007. PMID: 17609325 Free PMC article.
-
Genetic variability of the S gene of hepatitis B virus: clinical and diagnostic impact.J Clin Virol. 2005 Feb;32(2):102-12. doi: 10.1016/j.jcv.2004.10.008. J Clin Virol. 2005. PMID: 15653412 Review.
-
Hepatitis B vaccination.Hum Vaccin Immunother. 2015;11(1):53-7. doi: 10.4161/hv.34306. Epub 2014 Nov 1. Hum Vaccin Immunother. 2015. PMID: 25483515 Free PMC article. Review.
Cited by
-
Impact of the Interaction of Hepatitis B Virus with Mitochondria and Associated Proteins.Viruses. 2020 Feb 4;12(2):175. doi: 10.3390/v12020175. Viruses. 2020. PMID: 32033216 Free PMC article. Review.
-
Molecular Mechanisms during Hepatitis B Infection and the Effects of the Virus Variability.Viruses. 2021 Jun 18;13(6):1167. doi: 10.3390/v13061167. Viruses. 2021. PMID: 34207116 Free PMC article. Review.
-
Antigenic and conserved peptides from diverse Helicobacter pylori antigens.Biotechnol Lett. 2022 Mar;44(3):535-545. doi: 10.1007/s10529-022-03238-x. Epub 2022 Mar 11. Biotechnol Lett. 2022. PMID: 35277779 Free PMC article.
-
Recombinant HBsAg of the Wild-Type and the G145R Escape Mutant, included in the New Multivalent Vaccine against Hepatitis B Virus, Dramatically Differ in their Effects on Leukocytes from Healthy Donors In Vitro.Vaccines (Basel). 2022 Feb 3;10(2):235. doi: 10.3390/vaccines10020235. Vaccines (Basel). 2022. PMID: 35214692 Free PMC article.
-
Molecular evolution and genomics of hepatitis B virus subgenotype C2 strain predominant in the chronic patients in Bangladesh.Virusdisease. 2018 Dec;29(4):486-490. doi: 10.1007/s13337-018-0497-6. Epub 2018 Oct 17. Virusdisease. 2018. PMID: 30539051 Free PMC article.
References
-
- Seeger C MW, Zoulim F. Fields virology. Knipe DM HP, editor. Philadelphia, PA: Lippincott Williams & Wilkins; 2007.
-
- Locarnini S. Molecular virology of hepatitis B virus. Seminars in liver disease. 2004;24 Suppl 1:3–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

