Cytochrome c Negatively Regulates NLRP3 Inflammasomes
- PMID: 28030552
- PMCID: PMC5193325
- DOI: 10.1371/journal.pone.0167636
Cytochrome c Negatively Regulates NLRP3 Inflammasomes
Abstract
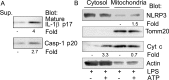
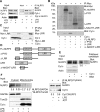
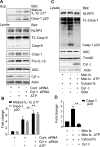
The release of cytochrome c from the inner mitochondrial membrane, where it is anchored by caridolipin, triggers the formation of the Apaf-1 apoptosome. Cardiolipin also interacts with NLRP3 recruiting NLRP3 to mitochondria and facilitating inflammasome assembly. In this study we investigated whether cytosolic cytochrome c impacts NLRP3 inflammasome activation in macrophages. We report that cytochrome c binds to the LRR domain of NLRP3 and that cytochrome c reduces the interactions between NLRP3 and cardiolipin and between NLRP3 and NEK7, a recently recognized component of the NLRP3 inflammasome needed for NLRP3 oligomerization. Protein transduction of cytochrome c impairs NLRP3 inflammasome activation, while partially silencing cytochrome c expression enhances it. The addition of cytochrome c to an in vitro inflammasome assay severely limited caspase-1 activation. We propose that there is a crosstalk between the NLRP3 inflammasome and apoptosome pathways mediated by cytochrome c, whose release during apoptosis acts to limit NLRP3 inflammasome activation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Glutathione Transferase Omega-1 Regulates NLRP3 Inflammasome Activation through NEK7 Deglutathionylation.Cell Rep. 2019 Oct 1;29(1):151-161.e5. doi: 10.1016/j.celrep.2019.08.072. Cell Rep. 2019. PMID: 31577945
-
NLRP3 phosphorylation in its LRR domain critically regulates inflammasome assembly.Nat Commun. 2021 Oct 6;12(1):5862. doi: 10.1038/s41467-021-26142-w. Nat Commun. 2021. PMID: 34615873 Free PMC article.
-
NEK7-Mediated Activation of NLRP3 Inflammasome Is Coordinated by Potassium Efflux/Syk/JNK Signaling During Staphylococcus aureus Infection.Front Immunol. 2021 Sep 16;12:747370. doi: 10.3389/fimmu.2021.747370. eCollection 2021. Front Immunol. 2021. PMID: 34603335 Free PMC article.
-
Recent advances in the NEK7-licensed NLRP3 inflammasome activation: Mechanisms, role in diseases and related inhibitors.J Autoimmun. 2020 Sep;113:102515. doi: 10.1016/j.jaut.2020.102515. Epub 2020 Jul 20. J Autoimmun. 2020. PMID: 32703754 Review.
-
Negative regulators and their mechanisms in NLRP3 inflammasome activation and signaling.Immunol Cell Biol. 2017 Aug;95(7):584-592. doi: 10.1038/icb.2017.23. Epub 2017 Mar 30. Immunol Cell Biol. 2017. PMID: 28356568 Review.
Cited by
-
NLRP3 inflammasome activation and cell death.Cell Mol Immunol. 2021 Sep;18(9):2114-2127. doi: 10.1038/s41423-021-00740-6. Epub 2021 Jul 28. Cell Mol Immunol. 2021. PMID: 34321623 Free PMC article. Review.
-
Computational Modeling of NLRP3 Identifies Enhanced ATP Binding and Multimerization in Cryopyrin-Associated Periodic Syndromes.Front Immunol. 2020 Nov 19;11:584364. doi: 10.3389/fimmu.2020.584364. eCollection 2020. Front Immunol. 2020. PMID: 33329557 Free PMC article.
-
How location and cellular signaling combine to activate the NLRP3 inflammasome.Cell Mol Immunol. 2022 Nov;19(11):1201-1214. doi: 10.1038/s41423-022-00922-w. Epub 2022 Sep 20. Cell Mol Immunol. 2022. PMID: 36127465 Free PMC article. Review.
-
Right place, right time: localisation and assembly of the NLRP3 inflammasome.F1000Res. 2019 May 17;8:F1000 Faculty Rev-676. doi: 10.12688/f1000research.18557.1. eCollection 2019. F1000Res. 2019. PMID: 31131091 Free PMC article. Review.
-
Swine Influenza Virus Induces RIPK1/DRP1-Mediated Interleukin-1 Beta Production.Viruses. 2018 Aug 9;10(8):419. doi: 10.3390/v10080419. Viruses. 2018. PMID: 30096906 Free PMC article.
References
-
- Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15(22):2922–33. Epub 2001/11/17. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

