Fusion of the dendritic cell-targeting chemokine MIP3α to melanoma antigen Gp100 in a therapeutic DNA vaccine significantly enhances immunogenicity and survival in a mouse melanoma model
- PMID: 28018602
- PMCID: PMC5168589
- DOI: 10.1186/s40425-016-0189-y
Fusion of the dendritic cell-targeting chemokine MIP3α to melanoma antigen Gp100 in a therapeutic DNA vaccine significantly enhances immunogenicity and survival in a mouse melanoma model
Abstract
Background: Although therapeutic cancer vaccines have been mostly disappointing in the clinic, the advent of novel immunotherapies and the future promise of neoantigen-based therapies have created the need for new vaccine modalities that can easily adapt to current and future developments in cancer immunotherapy. One such novel platform is a DNA vaccine fusing the chemokine Macrophage Inflammatory Protein-3α (MIP-3α) to an antigen, here melanoma antigen gp100. Previous published work has indicated that MIP-3α targets nascent peptides to immature dendritic cells, leading to processing by class I and II MHC pathways. This platform has shown enhanced efficacy in prophylactic melanoma and therapeutic lymphoma model systems.
Methods: The B16F10 melanoma syngeneic mouse model system was utilized, with a standard therapeutic protocol: challenge with lethal dose of B16F10 cells (5 × 104) on day 0 and then vaccinate by intramuscular electroporation with 50 μg plasmid on days three, 10, and 17. Efficacy was assessed by analysis of tumor burden, tumor growth, and mouse survival, using the statistical tests ANOVA, mixed effects regression, and log-rank, respectively. Immunogenicity was assessed by ELISA and flow cytometric methods, including intracellular cytokine staining to assess vaccine-specific T-cell responses, all tested by ANOVA.
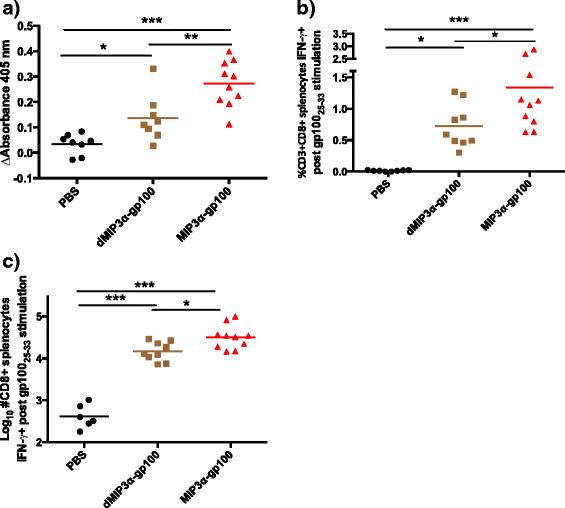
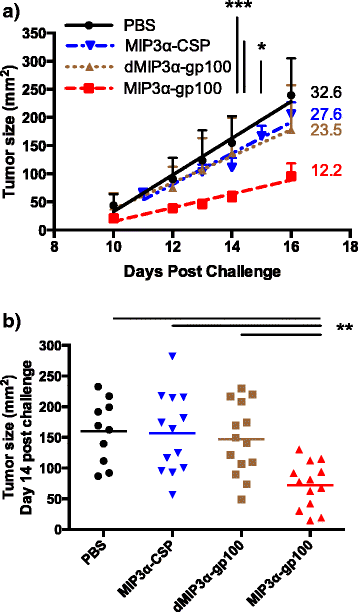
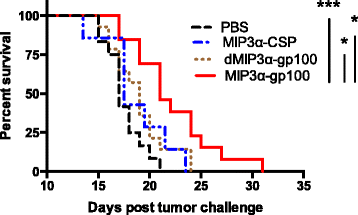
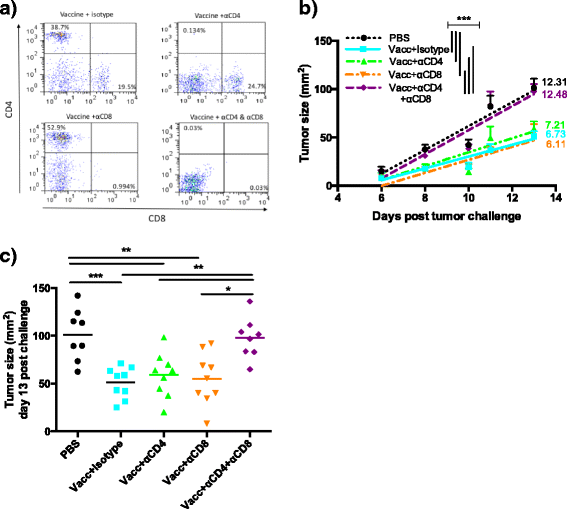
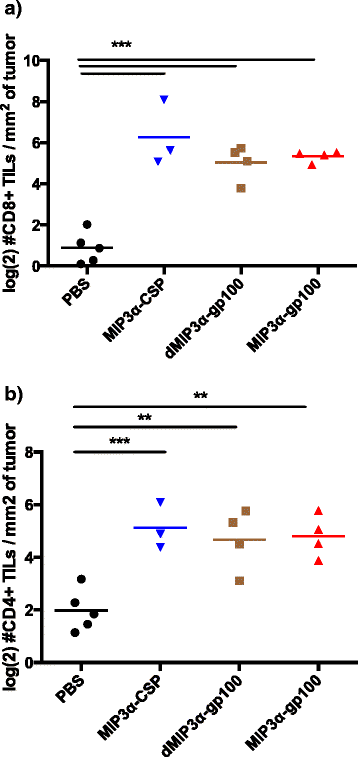
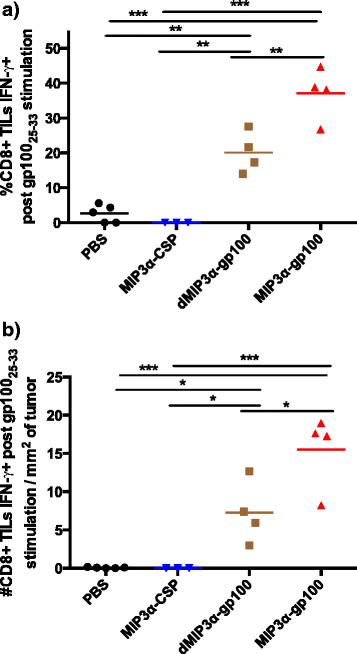
Results: We demonstrate that the addition of MIP3α to gp100 significantly enhances systemic anti-gp100 immunological parameters. Further, chemokine-fusion vaccine therapy significantly reduces tumor burden, slows tumor growth, and enhances mouse overall survival compared to antigen-only, irrelevant-antigen, and mock vaccines, with efficacy mediated by both CD4+ and CD8+ effector T cells. Antigen-only, irrelevant-antigen, and chemokine-fusion vaccines elicit significantly higher and similar CD4+ and CD8+ tumor-infiltrating lymphocyte (TIL) levels compared to mock vaccine. However, vaccine-specific CD8+ TILs are significantly higher in the chemokine-fusion vaccine group, indicating that the critical step induced by the fusion vaccine construct is the enhancement of vaccine-specific T-cell effectors.
Conclusions: The current study shows that fusion of MIP3α to melanoma antigen gp100 enhances the immunogenicity and efficacy of a DNA vaccine in a therapeutic B16F10 mouse melanoma model. This study analyzes an adaptable and easily produced MIP3α-antigen modular vaccine platform that could lend itself to a variety of functionalities, including combination treatments and neoantigen vaccination in the pursuit of personalized cancer therapy.
Keywords: B16 Melanoma; Chemokine-antigen fusion; DNA Vaccine; Gp100; In vivo electroporation; MIP3α, MIP3alpha, or CCL20; Therapeutic cancer vaccine.
Figures
Similar articles
-
Anti-IL-10-mediated Enhancement of Antitumor Efficacy of a Dendritic Cell-targeting MIP3α-gp100 Vaccine in the B16F10 Mouse Melanoma Model Is Dependent on Type I Interferons.J Immunother. 2018 May;41(4):181-189. doi: 10.1097/CJI.0000000000000212. J Immunother. 2018. PMID: 29334492 Free PMC article.
-
Treatment with an immature dendritic cell-targeting vaccine supplemented with IFN-α and an inhibitor of DNA methylation markedly enhances survival in a murine melanoma model.Cancer Immunol Immunother. 2020 Apr;69(4):569-580. doi: 10.1007/s00262-019-02471-0. Epub 2020 Jan 24. Cancer Immunol Immunother. 2020. PMID: 31980915 Free PMC article.
-
A multi-trimeric fusion of CD40L and gp100 tumor antigen activates dendritic cells and enhances survival in a B16-F10 melanoma DNA vaccine model.Vaccine. 2015 Sep 11;33(38):4798-806. doi: 10.1016/j.vaccine.2015.07.081. Epub 2015 Aug 1. Vaccine. 2015. PMID: 26241951 Free PMC article.
-
Nanomaterial-based delivery vehicles for therapeutic cancer vaccine development.Cancer Biol Med. 2021 May 12;18(2):352-71. doi: 10.20892/j.issn.2095-3941.2021.0004. Online ahead of print. Cancer Biol Med. 2021. PMID: 33979069 Free PMC article. Review.
-
Interest of Tumor-Specific CD4 T Helper 1 Cells for Therapeutic Anticancer Vaccine.Vaccines (Basel). 2015 Jun 30;3(3):490-502. doi: 10.3390/vaccines3030490. Vaccines (Basel). 2015. PMID: 26350591 Free PMC article. Review.
Cited by
-
Targeting Cross-Presentation as a Route to Improve the Efficiency of Peptide-Based Cancer Vaccines.Cancers (Basel). 2021 Dec 8;13(24):6189. doi: 10.3390/cancers13246189. Cancers (Basel). 2021. PMID: 34944809 Free PMC article.
-
The Collective Effect of MIP-3α and FL Promotes Dendritic Cell Function Within the Immune Microenvironment of Murine Liver Cancer.Front Oncol. 2021 Mar 26;11:646527. doi: 10.3389/fonc.2021.646527. eCollection 2021. Front Oncol. 2021. Retraction in: Front Oncol. 2021 Aug 23;11:744389. doi: 10.3389/fonc.2021.744389 PMID: 33842360 Free PMC article. Retracted.
-
Neoantigen Cancer Vaccines: Generation, Optimization, and Therapeutic Targeting Strategies.Vaccines (Basel). 2022 Jan 26;10(2):196. doi: 10.3390/vaccines10020196. Vaccines (Basel). 2022. PMID: 35214655 Free PMC article. Review.
-
IFNα and 5-Aza-2'-deoxycytidine combined with a dendritic-cell targeting DNA vaccine alter tumor immune cell infiltration in the B16F10 melanoma model.Front Immunol. 2023 Jan 19;13:1074644. doi: 10.3389/fimmu.2022.1074644. eCollection 2022. Front Immunol. 2023. PMID: 36741387 Free PMC article.
-
A Cancer Cell Program Promotes T Cell Exclusion and Resistance to Checkpoint Blockade.Cell. 2018 Nov 1;175(4):984-997.e24. doi: 10.1016/j.cell.2018.09.006. Cell. 2018. PMID: 30388455 Free PMC article.
References
-
- Small EJ, Schellhammer PF, Higano CS, Redfern CH, Nemunaitis JJ, Valone FH, Verjee SS, Jones LA, Hershberg RM. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24:3089–94. doi: 10.1200/JCO.2005.04.5252. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
