Oxidative stress in cancer and fibrosis: Opportunity for therapeutic intervention with antioxidant compounds, enzymes, and nanoparticles
- PMID: 28012439
- PMCID: PMC5198743
- DOI: 10.1016/j.redox.2016.12.011
Oxidative stress in cancer and fibrosis: Opportunity for therapeutic intervention with antioxidant compounds, enzymes, and nanoparticles
Abstract
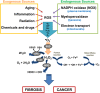
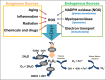
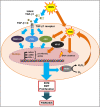
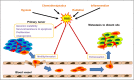
Oxidative stress, mainly contributed by reactive oxygen species (ROS), has been implicated in pathogenesis of several diseases. We review two primary examples; fibrosis and cancer. In fibrosis, ROS promote activation and proliferation of fibroblasts and myofibroblasts, activating TGF-β pathway in an autocrine manner. In cancer, ROS account for its genomic instability, resistance to apoptosis, proliferation, and angiogenesis. Importantly, ROS trigger cancer cell invasion through invadopodia formation as well as extravasation into a distant metastasis site. Use of antioxidant supplements, enzymes, and inhibitors for ROS-generating NADPH oxidases (NOX) is a logical therapeutic intervention for fibrosis and cancer. We review such attempts, progress, and challenges. Lastly, we review how nanoparticles with inherent antioxidant activity can also be a promising therapeutic option, considering their additional feature as a delivery platform for drugs, genes, and imaging agents.
Keywords: Antioxidant; Cancer; Fibrosis; Metastasis; Nanoparticles; ROS.
Copyright © 2016 The Authors. Published by Elsevier B.V. All rights reserved.
Figures

Similar articles
-
Role of NADPH oxidases in the redox biology of liver fibrosis.Redox Biol. 2015 Dec;6:106-111. doi: 10.1016/j.redox.2015.07.005. Epub 2015 Jul 14. Redox Biol. 2015. PMID: 26204504 Free PMC article. Review.
-
Trichostatin A inhibits transforming growth factor-β-induced reactive oxygen species accumulation and myofibroblast differentiation via enhanced NF-E2-related factor 2-antioxidant response element signaling.Mol Pharmacol. 2013 Mar;83(3):671-80. doi: 10.1124/mol.112.081059. Epub 2013 Jan 2. Mol Pharmacol. 2013. PMID: 23284002
-
Glucose-6-Phosphate Dehydrogenase Deficiency Activates Endothelial Cell and Leukocyte Adhesion Mediated via the TGFβ/NADPH Oxidases/ROS Signaling Pathway.Int J Mol Sci. 2020 Oct 10;21(20):7474. doi: 10.3390/ijms21207474. Int J Mol Sci. 2020. PMID: 33050491 Free PMC article.
-
Transforming growth factor β1 (TGFβ1)-induced CD44V6-NOX4 signaling in pathogenesis of idiopathic pulmonary fibrosis.J Biol Chem. 2017 Jun 23;292(25):10490-10519. doi: 10.1074/jbc.M116.752469. Epub 2017 Apr 7. J Biol Chem. 2017. PMID: 28389561 Free PMC article.
-
NADPH Oxidases NOXs and DUOXs as putative targets for cancer therapy.Anticancer Agents Med Chem. 2013 Mar;13(3):502-14. Anticancer Agents Med Chem. 2013. PMID: 22931418 Free PMC article. Review.
Cited by
-
Hydroxylated Fullerene: A Stellar Nanomedicine to Treat Lumbar Radiculopathy via Antagonizing TNF-α-Induced Ion Channel Activation, Calcium Signaling, and Neuropeptide Production.ACS Biomater Sci Eng. 2018 Jan 8;4(1):266-277. doi: 10.1021/acsbiomaterials.7b00735. Epub 2017 Dec 7. ACS Biomater Sci Eng. 2018. PMID: 30038959 Free PMC article.
-
Employing Supervised Algorithms for the Prediction of Nanomaterial's Antioxidant Efficiency.Int J Mol Sci. 2023 Feb 1;24(3):2792. doi: 10.3390/ijms24032792. Int J Mol Sci. 2023. PMID: 36769135 Free PMC article. Review.
-
Nanoantioxidants: Pioneer Types, Advantages, Limitations, and Future Insights.Molecules. 2021 Nov 21;26(22):7031. doi: 10.3390/molecules26227031. Molecules. 2021. PMID: 34834124 Free PMC article. Review.
-
Squid Ink Polysaccharides Protect Human Fibroblast Against Oxidative Stress by Regulating NADPH Oxidase and Connexin43.Front Pharmacol. 2020 Jan 17;10:1574. doi: 10.3389/fphar.2019.01574. eCollection 2019. Front Pharmacol. 2020. PMID: 32009967 Free PMC article.
-
Oxidative Stress Markers Patients with Parotid Gland Tumors: A Pilot Study.Biomed Res Int. 2018 Jan 30;2018:4340871. doi: 10.1155/2018/4340871. eCollection 2018. Biomed Res Int. 2018. PMID: 29651432 Free PMC article.
References
-
- Liu Y., Fiskum G., Schubert D. Generation of reactive oxygen species by the mitochondrial electron transport chain. J. Neurochem. 2002;80(5):780–787. - PubMed
-
- Bedard K., Krause K.H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87(1):245–313. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

