Benefits of Hormone Therapy Estrogens Depend on Estrogen Type: 17β-Estradiol and Conjugated Equine Estrogens Have Differential Effects on Cognitive, Anxiety-Like, and Depressive-Like Behaviors and Increase Tryptophan Hydroxylase-2 mRNA Levels in Dorsal Raphe Nucleus Subregions
- PMID: 28008302
- PMCID: PMC5143618
- DOI: 10.3389/fnins.2016.00517
Benefits of Hormone Therapy Estrogens Depend on Estrogen Type: 17β-Estradiol and Conjugated Equine Estrogens Have Differential Effects on Cognitive, Anxiety-Like, and Depressive-Like Behaviors and Increase Tryptophan Hydroxylase-2 mRNA Levels in Dorsal Raphe Nucleus Subregions
Abstract
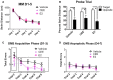
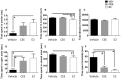
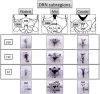
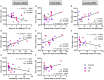
Decreased serotonin (5-HT) function is associated with numerous cognitive and affective disorders. Women are more vulnerable to these disorders and have a lower rate of 5-HT synthesis than men. Serotonergic neurons in the dorsal raphe nucleus (DRN) are a major source of 5-HT in the forebrain and play a critical role in regulation of stress-related disorders. In particular, polymorphisms of tryptophan hydroxylase-2 (TpH2, the brain-specific, rate-limiting enzyme for 5-HT biosynthesis) are implicated in cognitive and affective disorders. Administration of 17β-estradiol (E2), the most potent naturally circulating estrogen in women and rats, can have beneficial effects on cognitive, anxiety-like, and depressive-like behaviors. Moreover, E2 increases TpH2 mRNA in specific subregions of the DRN. Although conjugated equine estrogens (CEE) are a commonly prescribed estrogen component of hormone therapy in menopausal women, there is a marked gap in knowledge regarding how CEE affects these behaviors and the brain 5-HT system. Therefore, we compared the effects of CEE and E2 treatments on behavior and TpH2 mRNA. Female Sprague-Dawley rats were ovariectomized, administered either vehicle, CEE, or E2 and tested on a battery of cognitive, anxiety-like, and depressive-like behaviors. The brains of these animals were subsequently analyzed for TpH2 mRNA. Both CEE and E2 exerted beneficial behavioral effects, although efficacy depended on the distinct behavior and for cognition, on the task difficulty. Compared to CEE, E2 generally had more robust anxiolytic and antidepressant effects. E2 increased TpH2 mRNA in the caudal and mid DRN, corroborating previous findings. However, CEE increased TpH2 mRNA in the caudal and rostral, but not the mid, DRN, suggesting that distinct estrogens can have subregion-specific effects on TpH2 gene expression. We also found differential correlations between the level of TpH2 mRNA in specific DRN subregions and behavior, depending on the type of behavior. These distinct associations imply that cognition, anxiety-like, and depressive-like behaviors are modulated by unique serotonergic neurocircuitry, opening the possibility of novel avenues of targeted treatment for different types of cognitive and affective disorders.
Keywords: Premarin; TpH2; cognition; estrogen; learning and memory; mood; serotonin; spatial.
Figures

Similar articles
-
Estrogen receptor beta regulates the expression of tryptophan-hydroxylase 2 mRNA within serotonergic neurons of the rat dorsal raphe nuclei.Neuroscience. 2009 Oct 6;163(2):705-18. doi: 10.1016/j.neuroscience.2009.06.046. Epub 2009 Jun 23. Neuroscience. 2009. PMID: 19559077 Free PMC article.
-
Estrogen selectively increases tryptophan hydroxylase-2 mRNA expression in distinct subregions of rat midbrain raphe nucleus: association between gene expression and anxiety behavior in the open field.Biol Psychiatry. 2006 Aug 1;60(3):288-95. doi: 10.1016/j.biopsych.2005.10.019. Epub 2006 Feb 3. Biol Psychiatry. 2006. PMID: 16458260
-
Estrogen decreases 5-HT1B autoreceptor mRNA in selective subregion of rat dorsal raphe nucleus: inverse association between gene expression and anxiety behavior in the open field.Neuroscience. 2009 Jan 23;158(2):456-64. doi: 10.1016/j.neuroscience.2008.10.016. Epub 2008 Nov 1. Neuroscience. 2009. PMID: 19049819 Free PMC article.
-
Serotonergic systems, anxiety, and affective disorder: focus on the dorsomedial part of the dorsal raphe nucleus.Ann N Y Acad Sci. 2008 Dec;1148:86-94. doi: 10.1196/annals.1410.004. Ann N Y Acad Sci. 2008. PMID: 19120094 Review.
-
Reprint of: From the 90׳s to now: A brief historical perspective on more than two decades of estrogen neuroprotection.Brain Res. 2016 Aug 15;1645:79-82. doi: 10.1016/j.brainres.2016.06.016. Epub 2016 Jun 16. Brain Res. 2016. PMID: 27317847 Free PMC article. Review.
Cited by
-
Menopause, hormone therapy and cognition: maximizing translation from preclinical research.Climacteric. 2021 Aug;24(4):373-381. doi: 10.1080/13697137.2021.1917538. Epub 2021 May 12. Climacteric. 2021. PMID: 33977823 Free PMC article. Review.
-
Age Impacts the Burden That Reference Memory Imparts on an Increasing Working Memory Load and Modifies Relationships With Cholinergic Activity.Front Behav Neurosci. 2021 Feb 10;15:610078. doi: 10.3389/fnbeh.2021.610078. eCollection 2021. Front Behav Neurosci. 2021. PMID: 33643006 Free PMC article.
-
Estrogen, the Peripheral Immune System and Major Depression - A Reproductive Lifespan Perspective.Front Behav Neurosci. 2022 Apr 15;16:850623. doi: 10.3389/fnbeh.2022.850623. eCollection 2022. Front Behav Neurosci. 2022. PMID: 35493954 Free PMC article. Review.
-
Modulation of Tryptophan and Serotonin Metabolism as a Biochemical Basis of the Behavioral Effects of Use and Withdrawal of Androgenic-Anabolic Steroids and Other Image- and Performance-Enhancing Agents.Int J Tryptophan Res. 2018 Feb 19;11:1178646917753422. doi: 10.1177/1178646917753422. eCollection 2018. Int J Tryptophan Res. 2018. PMID: 29487480 Free PMC article.
-
Evaluations of memory, anxiety, and the growth factor IGF-1R after post-surgical menopause treatment with a highly selective progestin.Behav Brain Res. 2023 Jun 25;448:114442. doi: 10.1016/j.bbr.2023.114442. Epub 2023 Apr 20. Behav Brain Res. 2023. PMID: 37085118 Free PMC article.
References
-
- Acosta J. I., Mayer L., Talboom J. S., Tsang C. W., Smith C. J., Enders C. K., et al. . (2009). Transitional versus surgical menopause in a rodent model: etiology of ovarian hormone loss impacts memory and the acetylcholine system. Endocrinology 150, 4248–4259. 10.1210/en.2008-1802 - DOI - PMC - PubMed
-
- Alves S. E., Lopez V., McEwen B. S., Weiland N. G. (1998). Differential colocalization of estrogen receptor beta (ERbeta) with oxytocin and vasopressin in the paraventricular and supraoptic nuclei of the female rat brain: an immunocytochemical study. Proc. Natl. Acad. Sci. U.S.A. 95, 3281–3286. 10.1073/pnas.95.6.3281 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

