A20 Curtails Primary but Augments Secondary CD8+ T Cell Responses in Intracellular Bacterial Infection
- PMID: 28004776
- PMCID: PMC5177869
- DOI: 10.1038/srep39796
A20 Curtails Primary but Augments Secondary CD8+ T Cell Responses in Intracellular Bacterial Infection
Abstract
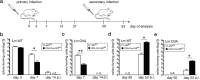
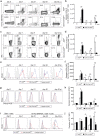
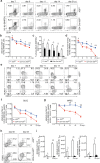
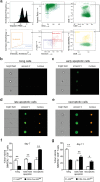
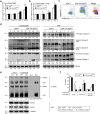
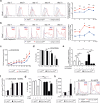
The ubiquitin-modifying enzyme A20, an important negative feedback regulator of NF-κB, impairs the expansion of tumor-specific CD8+ T cells but augments the proliferation of autoimmune CD4+ T cells. To study the T cell-specific function of A20 in bacterial infection, we infected T cell-specific A20 knockout (CD4-Cre A20fl/fl) and control mice with Listeria monocytogenes. A20-deficient pathogen-specific CD8+ T cells expanded stronger resulting in improved pathogen control at day 7 p.i. Imaging flow cytometry revealed that A20-deficient Listeria-specific CD8+ T cells underwent increased apoptosis and necroptosis resulting in reduced numbers of memory CD8+ T cells. In contrast, the primary CD4+ T cell response was A20-independent. Upon secondary infection, the increase and function of pathogen-specific CD8+ T cells, as well as pathogen control were significantly impaired in CD4-Cre A20fl/fl mice. In vitro, apoptosis and necroptosis of Listeria-specific A20-deficient CD8+ T cells were strongly induced as demonstrated by increased caspase-3/7 activity, RIPK1/RIPK3 complex formation and more morphologically apoptotic and necroptotic CD8+ T cells. In vitro, A20 limited CD95L and TNF-induced caspase3/7 activation. In conclusion, T cell-specific A20 limited the expansion but reduced apoptosis and necroptosis of Listeria-specific CD8+ T cells, resulting in an impaired pathogen control in primary but improved clearance in secondary infection.
Figures
Similar articles
-
Constitutive expression of IL-7 receptor alpha does not support increased expansion or prevent contraction of antigen-specific CD4 or CD8 T cells following Listeria monocytogenes infection.J Immunol. 2008 Mar 1;180(5):2855-62. doi: 10.4049/jimmunol.180.5.2855. J Immunol. 2008. PMID: 18292507
-
Protective immunosurveillance of the central nervous system by Listeria-specific CD4 and CD8 T cells in systemic listeriosis in the absence of intracerebral Listeria.J Immunol. 2002 Aug 15;169(4):2010-9. doi: 10.4049/jimmunol.169.4.2010. J Immunol. 2002. PMID: 12165527
-
Chronic ethanol induces inhibition of antigen-specific CD8+ but not CD4+ immunodominant T cell responses following Listeria monocytogenes inoculation.J Leukoc Biol. 2009 Jan;85(1):34-43. doi: 10.1189/jlb.0208101. Epub 2008 Sep 26. J Leukoc Biol. 2009. PMID: 18820175 Free PMC article.
-
Listeria monocytogenes: a model pathogen to study antigen-specific memory CD8 T cell responses.Semin Immunopathol. 2015 May;37(3):301-10. doi: 10.1007/s00281-015-0477-5. Epub 2015 Apr 10. Semin Immunopathol. 2015. PMID: 25860798 Free PMC article. Review.
-
T memory cells: quality not quantity.Curr Biol. 2002 Mar 5;12(5):R174-6. doi: 10.1016/s0960-9822(02)00735-2. Curr Biol. 2002. PMID: 11882307 Review.
Cited by
-
Deubiquitinating Enzyme: A Potential Secondary Checkpoint of Cancer Immunity.Front Oncol. 2020 Aug 7;10:1289. doi: 10.3389/fonc.2020.01289. eCollection 2020. Front Oncol. 2020. PMID: 32850399 Free PMC article. Review.
-
NF-κB regulated expression of A20 controls IKK dependent repression of RIPK1 induced cell death in activated T cells.Cell Death Differ. 2024 Sep 26. doi: 10.1038/s41418-024-01383-6. Online ahead of print. Cell Death Differ. 2024. PMID: 39327505
-
Mistuned NF-κB signaling in lymphocytes: lessons from relevant inborn errors of immunity.Clin Exp Immunol. 2023 Apr 25;212(2):117-128. doi: 10.1093/cei/uxad006. Clin Exp Immunol. 2023. PMID: 36651621 Free PMC article.
-
Topical Application of Temperature-Sensitive Gel Containing Caerin 1.1 and 1.9 Peptides on TC-1 Tumour-Bearing Mice Induced High-Level Immune Response in the Tumour Microenvironment.Front Oncol. 2021 Nov 11;11:754770. doi: 10.3389/fonc.2021.754770. eCollection 2021. Front Oncol. 2021. PMID: 34858827 Free PMC article.
-
Deubiquitinase function of A20 maintains and repairs endothelial barrier after lung vascular injury.Cell Death Discov. 2018 May 16;4:60. doi: 10.1038/s41420-018-0056-3. eCollection 2018. Cell Death Discov. 2018. PMID: 29796309 Free PMC article.
References
-
- Kaech S. M., Hemby S., Kersh E. & Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell 111, 837–851 (2002). - PubMed
-
- Hettmann T., Opferman J. T., Leiden J. M. & Ashton-Rickardt P. G. A critical role for NF-κB transcription factors in the development of CD8+ memory-phenotype T cells. Immunol. Lett. 85, 297–300 (2003). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

