Ectopic expression of specific GA2 oxidase mutants promotes yield and stress tolerance in rice
- PMID: 27998028
- PMCID: PMC5466439
- DOI: 10.1111/pbi.12681
Ectopic expression of specific GA2 oxidase mutants promotes yield and stress tolerance in rice
Abstract
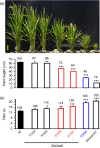
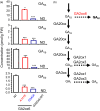
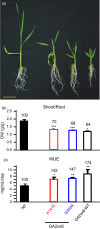
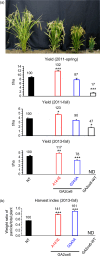
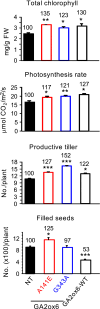
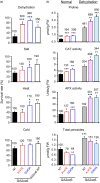
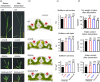
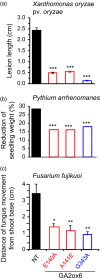
A major challenge of modern agricultural biotechnology is the optimization of plant architecture for enhanced productivity, stress tolerance and water use efficiency (WUE). To optimize plant height and tillering that directly link to grain yield in cereals and are known to be tightly regulated by gibberellins (GAs), we attenuated the endogenous levels of GAs in rice via its degradation. GA 2-oxidase (GA2ox) is a key enzyme that inactivates endogenous GAs and their precursors. We identified three conserved domains in a unique class of C20 GA2ox, GA2ox6, which is known to regulate the architecture and function of rice plants. We mutated nine specific amino acids in these conserved domains and observed a gradient of effects on plant height. Ectopic expression of some of these GA2ox6 mutants moderately lowered GA levels and reprogrammed transcriptional networks, leading to reduced plant height, more productive tillers, expanded root system, higher WUE and photosynthesis rate, and elevated abiotic and biotic stress tolerance in transgenic rice. Combinations of these beneficial traits conferred not only drought and disease tolerance but also increased grain yield by 10-30% in field trials. Our studies hold the promise of manipulating GA levels to substantially improve plant architecture, stress tolerance and grain yield in rice and possibly in other major crops.
Keywords: GA 2 oxidase 6; gibberellin; photosynthesis rate; plant architecture; rice; stress tolerance; yield.
© 2016 The Authors. Plant Biotechnology Journal published by Society for Experimental Biology and The Association of Applied Biologists and John Wiley & Sons Ltd.
Figures

Similar articles
-
An A20/AN1-type zinc finger protein modulates gibberellins and abscisic acid contents and increases sensitivity to abiotic stress in rice (Oryza sativa).J Exp Bot. 2016 Jan;67(1):315-26. doi: 10.1093/jxb/erv464. Epub 2015 Oct 27. J Exp Bot. 2016. PMID: 26512055
-
The BEL1-like homeodomain protein OsBLH4 regulates rice plant height, grain number, and heading date by repressing the expression of OsGA2ox1.Plant J. 2024 Aug;119(3):1369-1385. doi: 10.1111/tpj.16857. Epub 2024 Jun 2. Plant J. 2024. PMID: 38824648
-
Cytokinin oxidase2-deficient mutants improve panicle and grain architecture through cytokinin accumulation and enhance drought tolerance in indica rice.Plant Cell Rep. 2024 Aug 3;43(8):207. doi: 10.1007/s00299-024-03289-6. Plant Cell Rep. 2024. PMID: 39096362
-
Phytohormones and rice crop yield: strategies and opportunities for genetic improvement.Transgenic Res. 2006 Aug;15(4):399-404. doi: 10.1007/s11248-006-0024-1. Transgenic Res. 2006. PMID: 16906440 Review.
-
Finding the genes to build C4 rice.Curr Opin Plant Biol. 2016 Jun;31:44-50. doi: 10.1016/j.pbi.2016.03.012. Epub 2016 Apr 4. Curr Opin Plant Biol. 2016. PMID: 27055266 Review.
Cited by
-
Targeted suppression of gibberellin biosynthetic genes ZmGA20ox3 and ZmGA20ox5 produces a short stature maize ideotype.Plant Biotechnol J. 2022 Jun;20(6):1140-1153. doi: 10.1111/pbi.13797. Epub 2022 Mar 9. Plant Biotechnol J. 2022. PMID: 35244326 Free PMC article.
-
Modulating root system architecture: cross-talk between auxin and phytohormones.Front Plant Sci. 2024 Feb 8;15:1343928. doi: 10.3389/fpls.2024.1343928. eCollection 2024. Front Plant Sci. 2024. PMID: 38390293 Free PMC article. Review.
-
Genome-wide identification of GA2ox genes family and analysis of PbrGA2ox1-mediated enhanced chlorophyll accumulation by promoting chloroplast development in pear.BMC Plant Biol. 2024 Mar 4;24(1):166. doi: 10.1186/s12870-024-04842-x. BMC Plant Biol. 2024. PMID: 38433195 Free PMC article.
-
Phytohormones enhanced drought tolerance in plants: a coping strategy.Environ Sci Pollut Res Int. 2018 Nov;25(33):33103-33118. doi: 10.1007/s11356-018-3364-5. Epub 2018 Oct 3. Environ Sci Pollut Res Int. 2018. PMID: 30284160 Review.
-
Overexpression of Jatropha Gibberellin 2-oxidase 6 (JcGA2ox6) Induces Dwarfism and Smaller Leaves, Flowers and Fruits in Arabidopsis and Jatropha.Front Plant Sci. 2017 Dec 12;8:2103. doi: 10.3389/fpls.2017.02103. eCollection 2017. Front Plant Sci. 2017. PMID: 29312375 Free PMC article.
References
-
- Achard, P. , Renou, J.P. , Berthome, R. , Harberd, N.P. and Genschik, P. (2008) Plant DELLAs restrain growth and promote survival of adversity by reducing the levels of reactive oxygen species. Curr. Biol. 18, 656–660. - PubMed
-
- Apel, K. and Hirt, H. (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55, 373–399. - PubMed
-
- Bates, L.S. , Waldren, R.P. and Teare, I.D. (1973) Rapid determination of free proline for water‐stress studies. Plant Soil 39, 205–207.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

